Preparation method for trace tritiated deoxynivalenol
A deoxynivalenol and labeling technology, which is applied in the direction of organic chemistry, can solve the problems of not being able to determine the number of 14C, not conforming to the trace research of such compounds, and the qualitative and quantitative tests cannot be carried out, etc., to achieve the reaction The conditions are easy to control, the synthesis conditions are suitable, and the cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
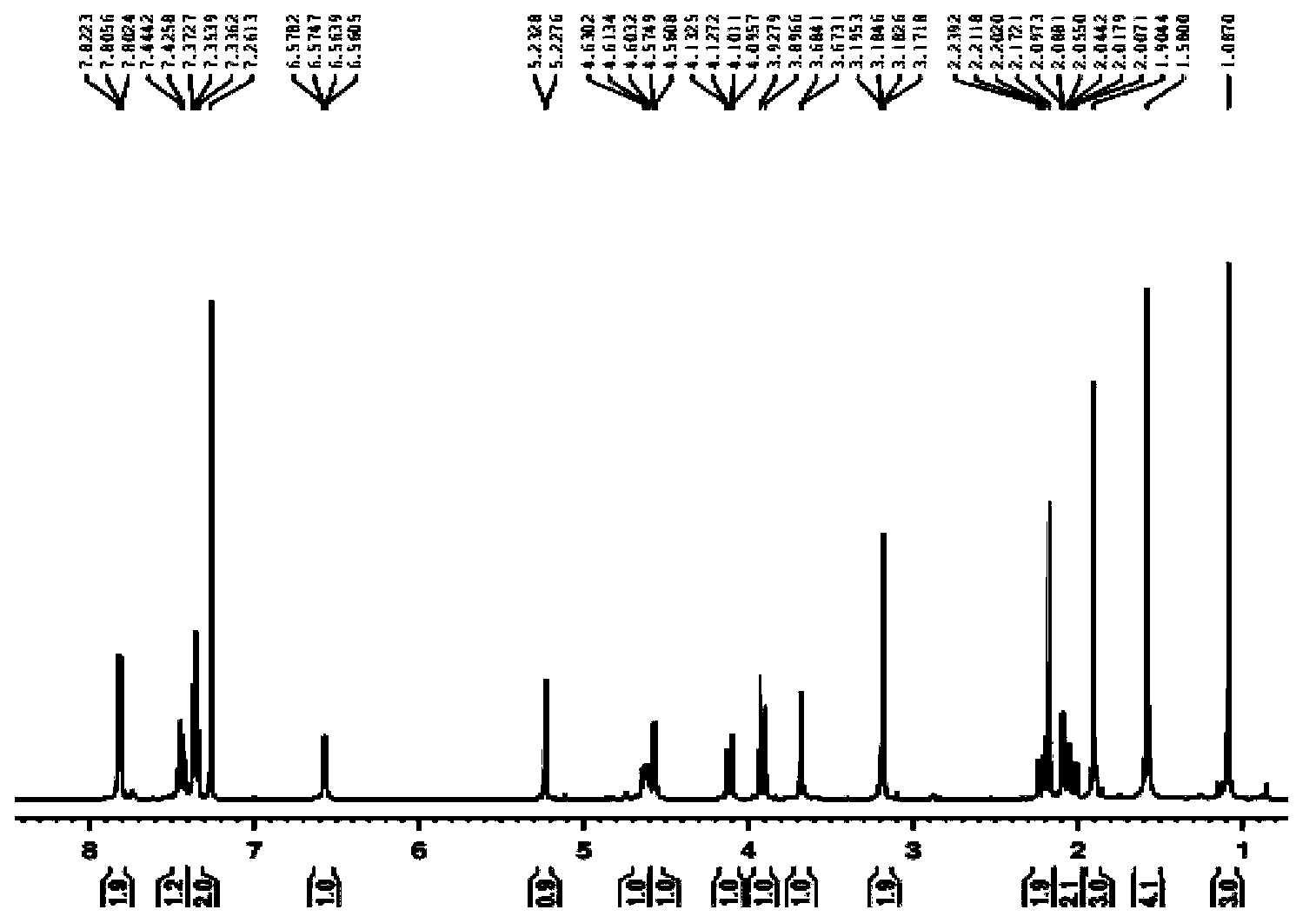
Image
Examples
Embodiment 1
[0039] Take 400mg of deoxynivalenol (content 70%, purity >90%), 300mg of phenylboronic acid, and appropriate amount of activated molecular sieve (4A). After adding anhydrous acetone, stopper and seal to isolate the air. After reacting for 4 h, the molecular sieves were removed by filtration under reduced pressure. Crystallization with acetone:petroleum ether (v / v 4:1) gave PBA-DON as a white needle-like solid.
[0040] Dissolve 20mg of PBA-DON in 1mL of anhydrous dichloromethane, seal, dry, and set aside (1); dissolve 30μL of oxalyl chloride in 2mL of anhydrous dichloromethane, pre-cool to -60°C, and set aside (2) ; Dissolve 50 μL of anhydrous dimethyl sulfoxide (DMSO) in 2 mL of anhydrous dichloromethane for use in (3); slowly add (3) to (2) dropwise, and stir for 30 minutes while adding at -78°C. A white solid (4) can be observed; slowly add (1) to (4), stir at -78°C for 30 min or 2 h, then add 200 μL of triethylamine, continue stirring for 5 min, add 10 mL of water to ter...
Embodiment 2
[0043] The operation method is the same as in Example 1, wherein: no activated molecular sieve is added to the phenylboronic acid protection reaction.
Embodiment 3
[0045] The protection reaction of phenylboronic acid is the same as in Examples 1 and 2. Oxidation reactions employ trifluoroacetic anhydride and DMSO as oxidants. The operation method is as follows.
[0046] Dissolve 50mg of PBA-DON in 6mL of anhydrous dichloromethane, seal, dry and set aside (1). Dissolve 30 μL of trifluoroacetic anhydride in 2 mL of anhydrous dichloromethane, pre-cool to -60 °C, and set aside (2). Dissolve 50 μL of anhydrous DMSO in 2 mL of anhydrous dichloromethane for use (3). (3) was slowly added dropwise to (2), and stirred at -60°C for 15 minutes, and a white solid (4) could be observed to form. Slowly add (1) to (4), stir at -60°C for 30 minutes, add 200 μL triethylamine, continue stirring for 5 minutes, add 10 mL water to terminate the reaction, extract the organic layer with ethyl acetate three times, combine the ethyl acetate layers, Wash with water first, then with saturated NaCl, and then dry with anhydrous sodium sulfate overnight. The next...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com