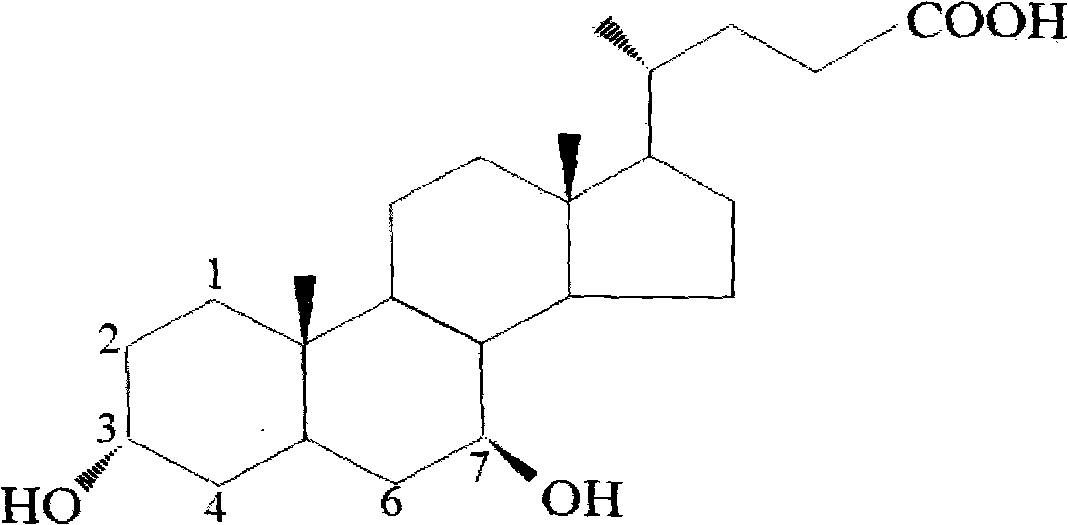
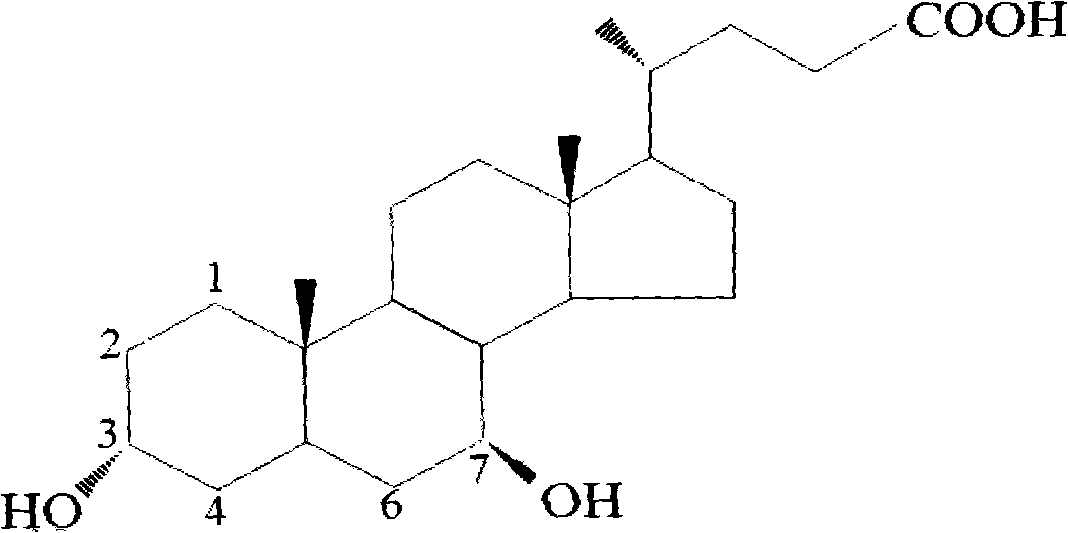
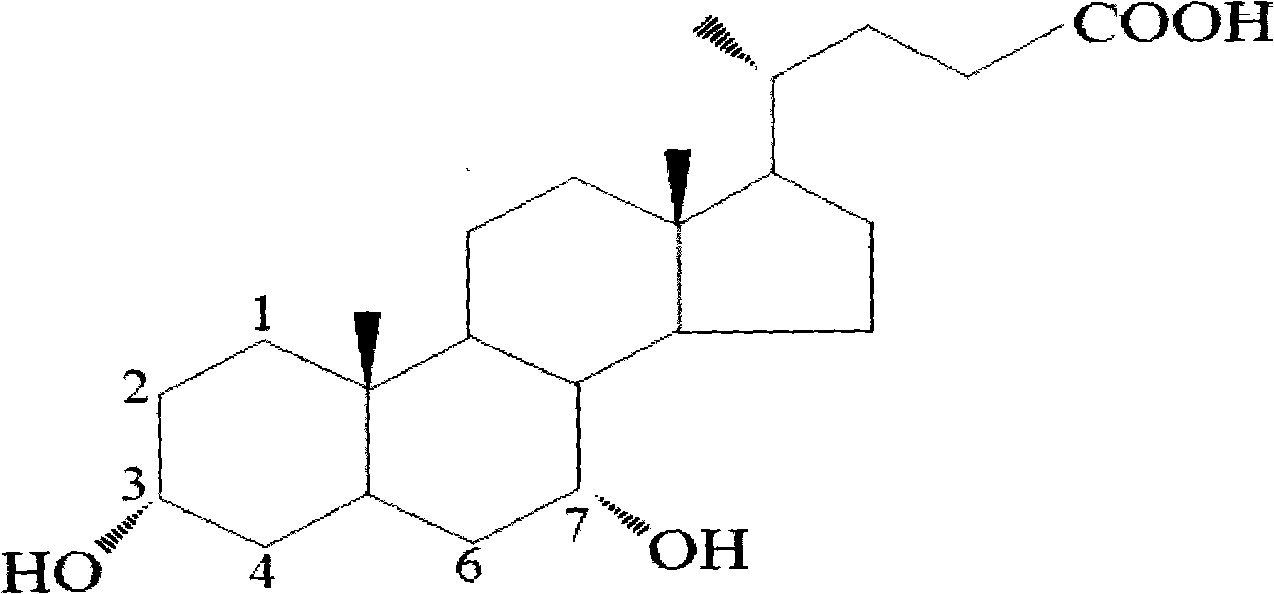
Preparation method of ursodesoxycholic acid
A technology of ursodeoxycholic acid and chenodeoxycholic acid, which is applied in the field of preparation of ursodeoxycholic acid, can solve the problems of low product yield and unprotected hydroxyl groups, and achieve high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] In a 2000 ml reaction flask, add 100 grams of chenodeoxycholic acid, 61 grams of maleic anhydride, 400 milliliters of acetone, 43.6 grams of diethylamine, and 2 grams of 2,6-lutidine. Raise the temperature to 38-40°C and maintain stirring at this temperature for 4-5 hours. After detecting the completeness of the reaction by TLC, 300 ml of water was added, and the acetone was removed by vacuum distillation to obtain a clear brown-yellow solution, which was cooled to 20-25°C. 10% hydrochloric acid was added dropwise to adjust the pH to 3.5-4.0, and turbidity appeared. After about 20 minutes, 300 ml of methanol was added, and crystallization occurred. Add 300 milliliters of water, and stir at 20-25 ° C for 2-3 hours, filter, wash with 200 milliliters of water, and dry to obtain 123.6 grams of white to off-white solid powder (formula III, 3a-ester-7a-hydroxy-5β-cholic acid , R-CDCA).
[0054] In a 2000 ml reaction flask, add 123.6 g of the product from the previous step,...
Embodiment 2
[0058] In a 2000ml reaction flask, add 100g of chenodeoxycholic acid, 59g of glutaric anhydride, 400ml of 3-methylfuran, 58.6g of tetramethylguanidine, heat up to 25-30°C, and Stirring was maintained for 7-9 hours. After detecting the completeness of the reaction by TLC, 300 ml of water was added, and the reactant was vacuum distilled to remove the organic solvent to obtain a brownish-yellow solution, which was cooled to 20-25°C. Add 10% hydrochloric acid dropwise to adjust the pH to 3.5-4.0. The temperature must be maintained below 30°C throughout the process, and turbidity occurs. After about 20 minutes, 220 ml of ethanol was added dropwise, and crystallization occurred. Continue to add 300 ml of water dropwise in 5-10 minutes, and stir at 20-25 ° C for 2-3 hours, filter, wash with 200 ml of water, and dry to obtain 127.4 grams of white to off-white solid powder (formula III, 3a-ester group-7a -hydroxy-5β-cholic acid, R-CDCA).
[0059] In a 2000 ml reaction flask, add 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com