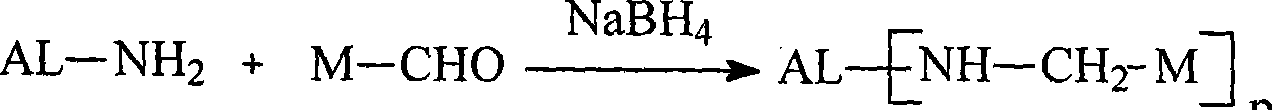Biological degradable albumin derivant, pharmacy composition, preparation and application of the same
A technology of albumin and derivatives, applied in the field of pharmaceutical preparations, can solve the problems of drug-induced rash, shortness of breath, bronchospasm, low solubility of paclitaxel, easy dissociation of micelles, etc., and achieve high drug loading and encapsulation efficiency , Good redispersibility, no particle adhesion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
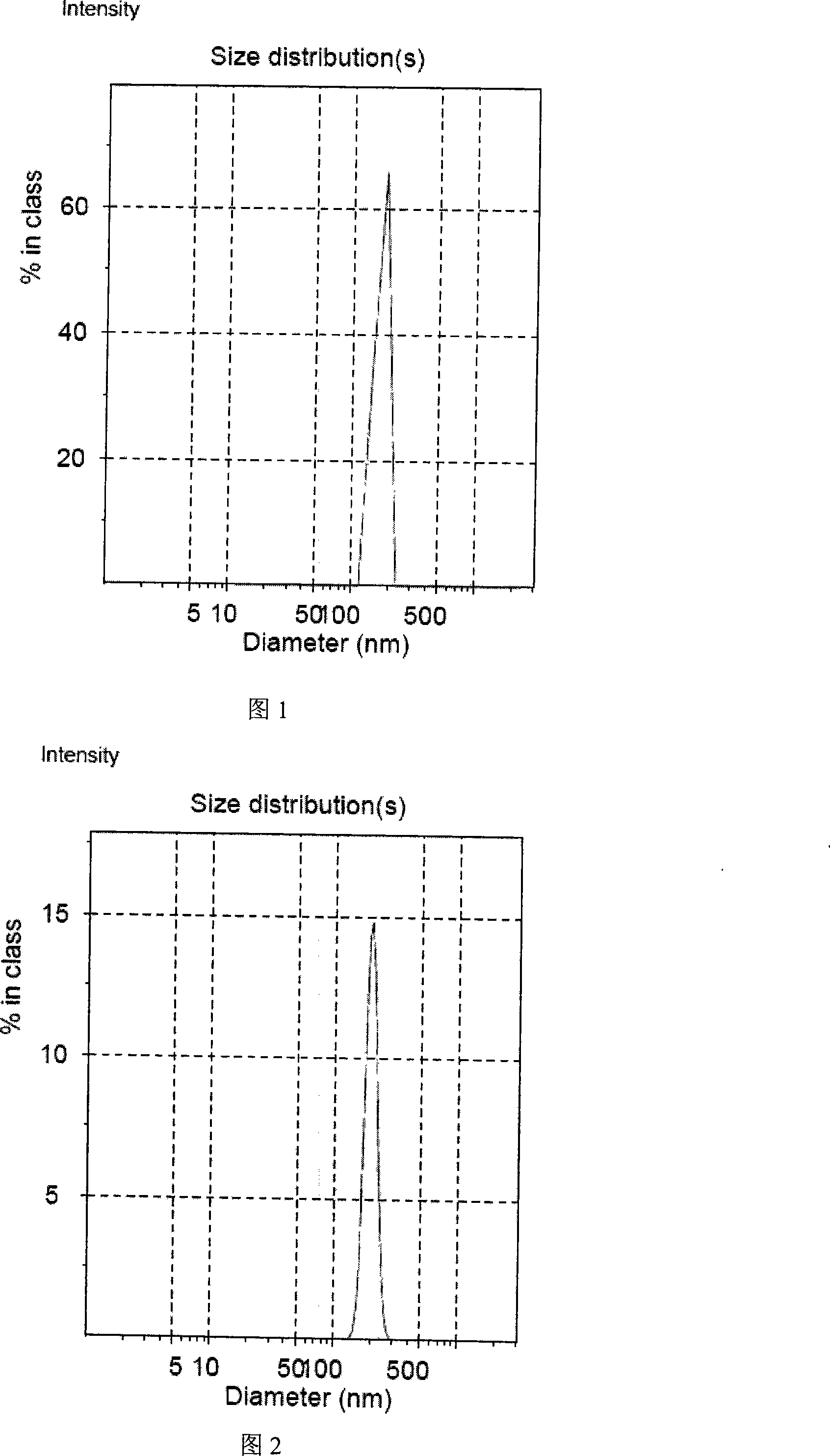
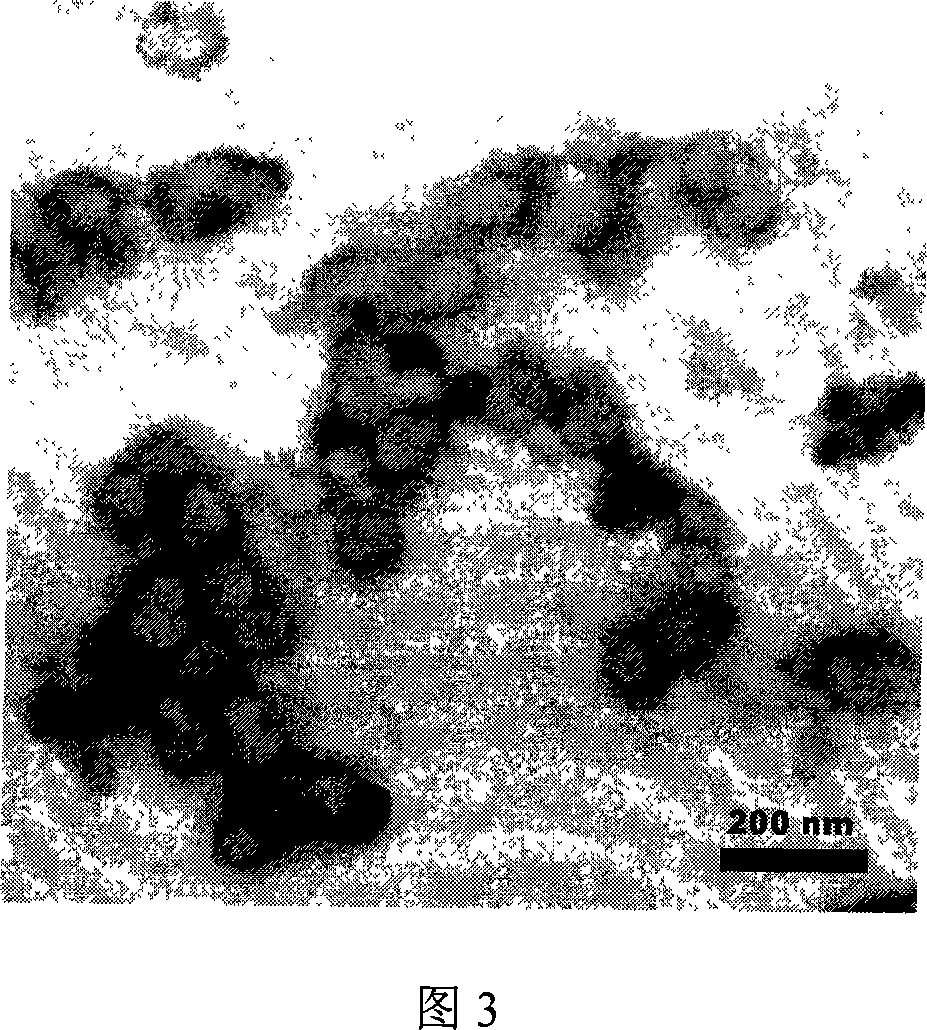
Image
Examples
Embodiment 1
[0097] Preparation of N-octyl human serum albumin
[0098] Dissolve 1g of human serum albumin in water, add 1.02g of n-octanal, react for 1h, add NaBH 4 The aqueous solution was continuously stirred for 12 hours, adjusted to pH 7 with HCl solution, dialyzed in distilled water for 3 days (MWCO=12000-14000), and freeze-dried to obtain the product. Elemental analysis shows that P is 35, that is, there are 35 free amino groups in an albumin molecular chain undergoing chemical reactions.
Embodiment 2
[0100] Preparation of N-octyl human serum albumin
[0101] The reaction conditions were the same as in Example 1, and the octanal reaction time was extended to 8 hours. The elemental analysis of the obtained product showed that P was 67, that is, there were 67 free amino groups in an albumin molecular chain to undergo chemical reactions.
Embodiment 3
[0103] Preparation of N-octanoyl human serum albumin
[0104] Add 3.6 g of n-octanoic acid (0.05 mol) to 4 ml of acetic anhydride, and distill off the acetic acid at 120° C. to obtain n-octanoic anhydride. Disperse 1 g of albumin in water / methanol, add n-octanoic anhydride dropwise under stirring, react at 40°C for 8 hours, dialyze the reaction solution, and freeze-dry to obtain the product. Elemental analysis shows that P is 24, that is, one albumin molecular chain has 24 free amino groups undergoing chemical reactions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com