Synthesis method of chenodeoxycholic acid
A technology of chenodeoxycholic acid and synthesis method, which is applied in the synthesis field of chenodeoxycholic acid, can solve the problems of incompatibility, low yield, complicated extraction and preparation process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
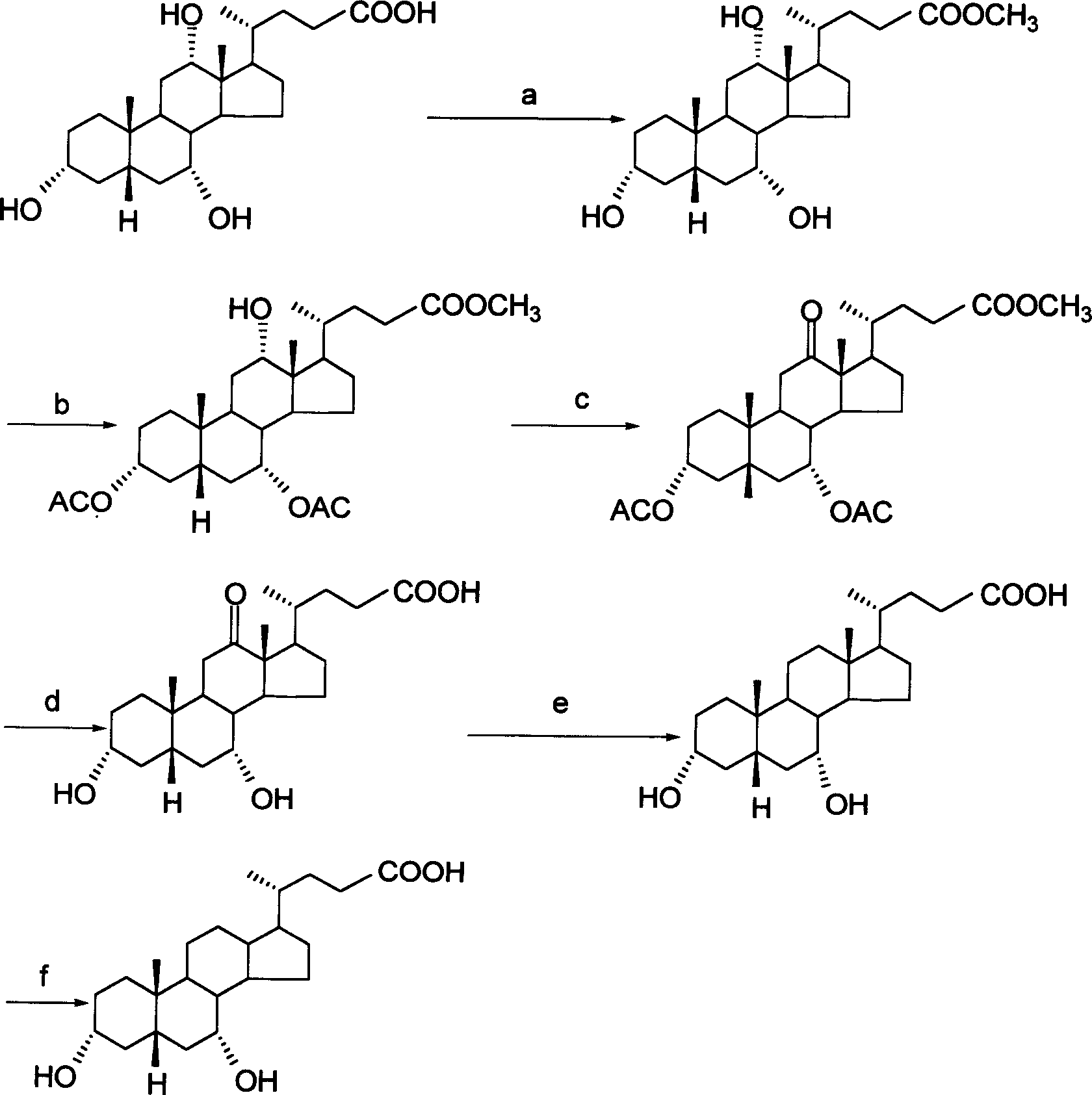
[0025] (1) Preparation of cholic acid ester
[0026] 50 grams of cholic acid was weighed, dissolved in 150 ml of anhydrous methanol, added with 5 ml of concentrated hydrochloric acid, refluxed for 30 minutes, slowly cooled and placed in a freezer, the yield of methyl cholic acid was 95%.
[0027] (2) Preparation of methyl diacetyl-12α-hydroxy-cholate
[0028] Weigh 50 grams of methyl cholic acid, dissolve it in 100 ml of refined pyridine, make it completely dissolved, add 100 ml of acetic anhydride under stirring, react at room temperature for 3 to 4 hours, pour it into 500 ml of water, there is a white precipitate, put it in. Put it in the refrigerator and filter the next day to obtain methyl diacetyl-12α-hydroxy-cholate with a yield of 40%.
[0029] (3) Preparation of 3α,7α-diacetoxy-12-oxo-methylcholanoic acid
[0030] Weigh 25 g of the crude product obtained above, dissolve it in 250 ml of acetone, filter to remove insoluble matter, slowly add Jones reagent under stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com