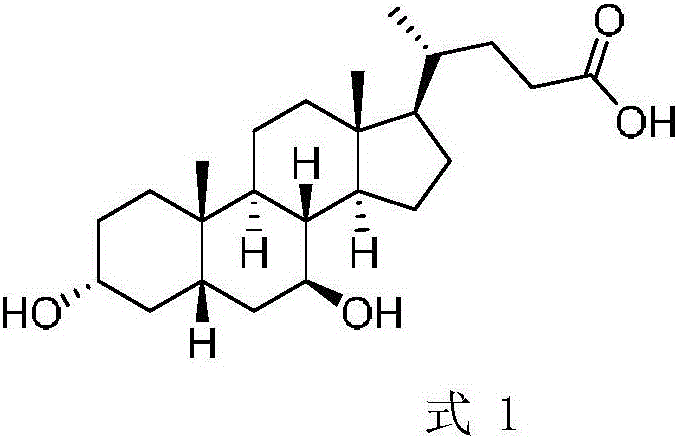
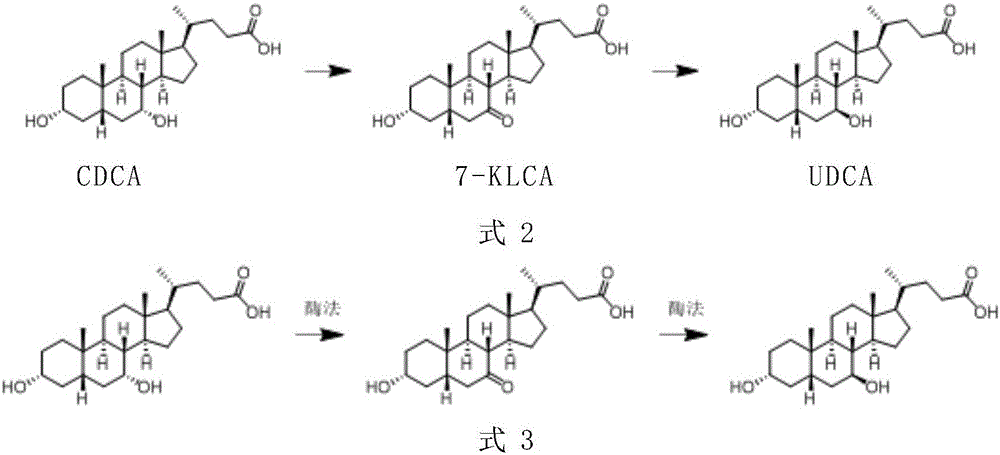
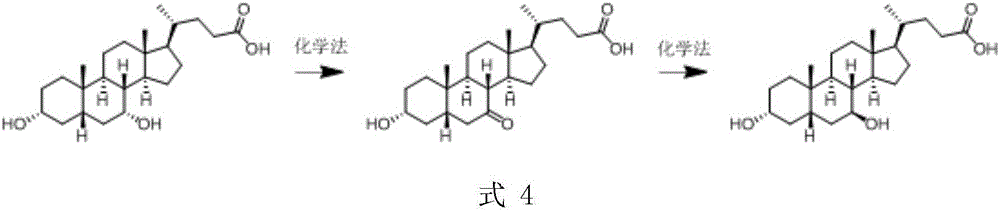
A chemical-enzyme method of preparing ursodeoxycholic acid
A technology for the preparation of ursodeoxycholic acid and enzymatic method, which is applied in the field of chemical-enzymatic preparation of ursodeoxycholic acid, can solve the problems of low product ee, increased cost, and reduced yield, and achieve low overall cost and improved The effect of ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 prepares 7-KLCA
[0026] Add 5 g of CDCA and 2 g of tartaric acid into 50 ml of methanol, and stir at room temperature for 10 minutes. Slowly add 25mL of sodium hypochlorite solution (active chlorine 5%) dropwise, and the dropwise addition is completed in 3 hours, and continue to stir for 5 hours. The reaction is monitored by HPLC, and the conversion rate is 99%. After the reaction, 0.5 g of sodium sulfite was added, after stirring, 200 mL of water was added, and 5 g of 7-KLCA was obtained by filtration with a content of 95%.
Embodiment 2
[0027] Embodiment 2 prepares UDCA
[0028] Add 5g 7-KLCA, 4g glucose monohydrate, 45mL 0.1M PBS buffer solution with pH 6.5 to a 100mL three-neck reaction flask, place in a 35°C water bath, and stir mechanically at 400-450rpm for 5min;
[0029] Add 5 mL of n-hexanol and 0.25 mL of Triton X-100 to the reaction flask, and after stirring evenly, add 0.1 g of 7-KLCA reductase (obtained by expressing sequence 20 in Escherichia coli) and glucose dehydrogenation Enzyme (from Suzhou Hanzyme Biotechnology Co., Ltd., No. EW002) 0.1g, NADP 0.005g, start the reaction, use 1M NaOH solution to control the pH at 6.5±0.1 during the reaction. After 4 hours, the reaction is completed, adjust the pH to 3.0, etc. The volume of ethyl acetate was extracted three times, the organic phase was combined, the pH was adjusted to 9.5, the equal volume of water was extracted three times, the aqueous phase was combined, and 4.7 g of the product was obtained by rotary evaporation. The product content was 95%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com