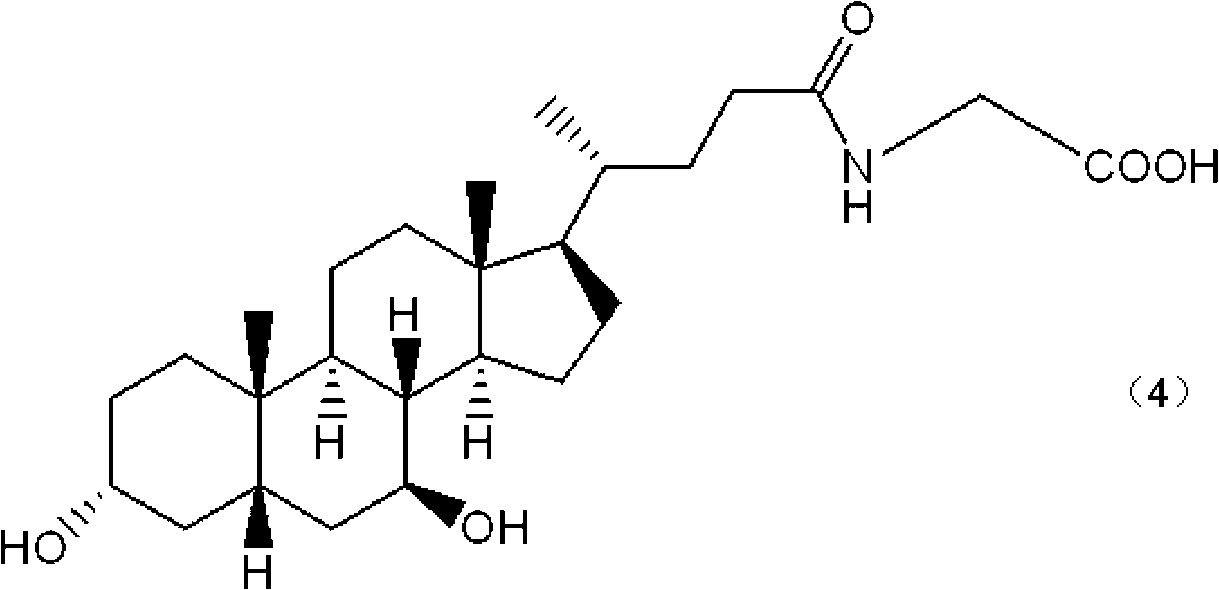
Method for preparing binding-form ursodesoxycholic acid by two-step enzymatic method
A technology of ursodeoxycholic acid and chenodeoxycholic acid, which is applied in the field of bound ursodeoxycholic acid, can solve problems such as difficulty in simulation, and achieve the effects of simple preparation method, high conversion rate, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0045] Implementation Case 1: Preparation of Chicken, Duck and Goose Gall Powder
[0046] Chicken gall, duck gall and goose gall are purchased from slaughterhouses. First determine the number and weight of gallbladder. Use 75% alcohol to sterilize parts of chicken gall, duck gall and goose gall respectively, and then dissect the bile separately. Put the bile taken out into different stainless steel round basins. Put the bile-filled disc into -40°C refrigerator for 24 hours, and finally put it into a freeze dryer and dry it for 48 hours to obtain chicken gall powder, duck gall powder, and goose gall powder respectively.
Embodiment example 2
[0047] Implementation Case 2: Content Analysis of TCDCA and TUDCA in Chicken Gall Powder, Duck Gall Powder and Goose Gall Powder
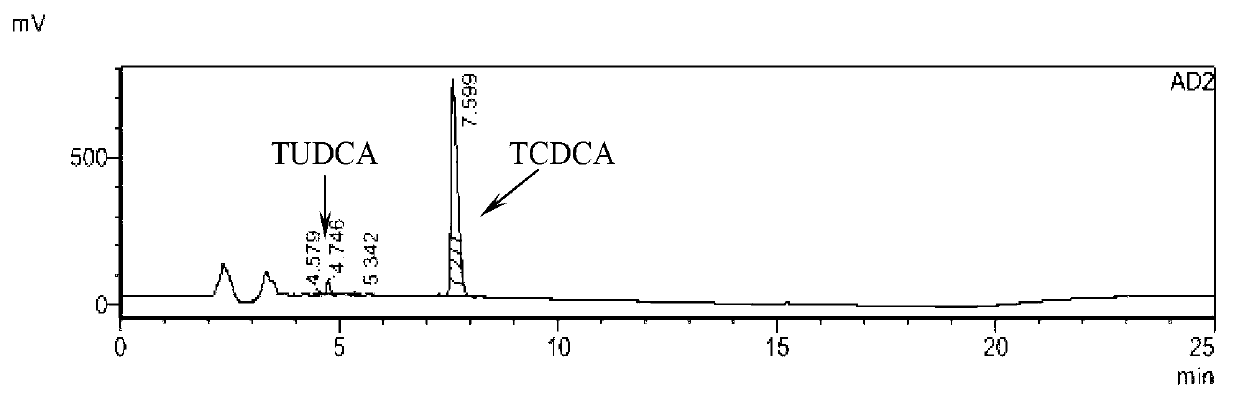
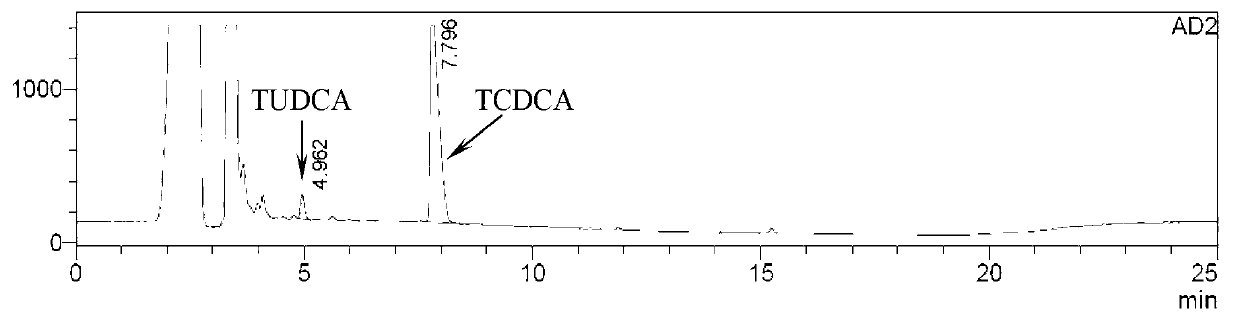
[0048] Take about 0.16g of chicken gall powder, duck gall powder, and goose gall powder, respectively, and place them in different measuring bottles (50mL). Dilute with methanol to the mark, shake well, filter, take the filtrate, that is. Take 10 μL of each of the above solutions and inject them into a high-performance liquid chromatograph, and the detector adopts an evaporative light scattering detector. Record the chromatogram ( figure 1 , figure 2 , image 3 ). The percentage contents of TCDCA and TUDCA were calculated respectively (Table 1). The reference substances TCDCA and TUDCA were from China Institute for the Control of Pharmaceutical and Biological Products.
[0049] Table 1 The contents of TCDCA and TUDCA in chicken gall powder, duck gall powder and goose gall powder detected by HPLC (%)
[0050]
Embodiment example 3
[0051] Example 3: Whole gene synthesis of 7α-HSDH and 7β-HSDH
[0052] The optimized 7α-HSDH gene sequence from Clostridium sardiniense (Clostridium sardiniense, ATCC27555) after gene sequence optimization is shown in SEQ ID No 1; The 7β-HSDH gene sequence is shown in SEQ ID No 2; the optimized 7α-HSDH gene sequence from Bacteroides fragilis ATCC25825) is shown in SEQ ID No 3; the optimized gene sequence is from the production The 7β-HSDH gene sequence of Collinsella aerofaciens ATCC25986 is shown in SEQ ID No 4. The above-mentioned codon-optimized gene sequence was synthesized by Sangon Biotech (Shanghai) Co., Ltd., and the restriction sites were both BamH I and Not I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com