Personalized delivery vector-based immunotherapy and uses thereof
A technology of immunotherapy and carrier, which is applied in the direction of carrier, nucleic acid carrier, gene therapy, etc., and can solve problems such as the difficulty of clarifying clinical benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0701] Methods of making these fragments are known in the art. (See, eg, Harlow and Lane, Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory, New York, 1988, which is incorporated herein by reference).
[0702] In some embodiments, antibody fragments can be prepared by proteolysis of antibodies or by expression of DNA encoding the fragments in E. coli or mammalian cells (eg, Chinese hamster egg cell culture or other protein expression systems).
[0703] In some embodiments, antibody fragments can be obtained by pepsin or papain digestion of whole antibodies by conventional methods. For example, antibody fragments can be obtained as F(ab') by enzymatic cleavage of antibodies with pepsin. 2 The indicated 5S fragment was prepared. This fragment can be further cleaved using a thiol reducing agent, and optionally a capping group using a thiol group obtained from cleavage of the disulfide bond, to generate a 3.5S Fab' monovalent fragment. Alternatively, enzymatic clea...
example 1-2
[1085] cell line
[1086] C57BL / 6 syngeneic TC-1 tumors were immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene. TC-1 provided by T.C. Wu (Johns Hopkins University School of Medicine, Baltimore, MD) are highly tumorigenic lung epithelial cells that express low levels of HPV-16 E6 and E7 and are transformed by the c-Ha-ras oncogene . Keep TC-1 at 37°C and 10% CO 2 Grow in RPMI 1640, 10% FCS, 2mM L-glutamine, 100U / ml penicillin, 100μg / ml streptomycin, 100μM non-essential amino acids, 1mM sodium pyruvate, 50 micromolar (mcM) 2-ME, 400 Micrograms (mcg) / mlG418 and 10% National Collection Type Culture-109 medium (National Collection Type Culture-109 medium). C3 are mouse embryonic cells from C57BL / 6 mice immortalized with the complete HPV 16 genome and transformed with pEJ-ras. EL-4 / E7 is thymoma EL-4 transduced with E7 retrovirus.
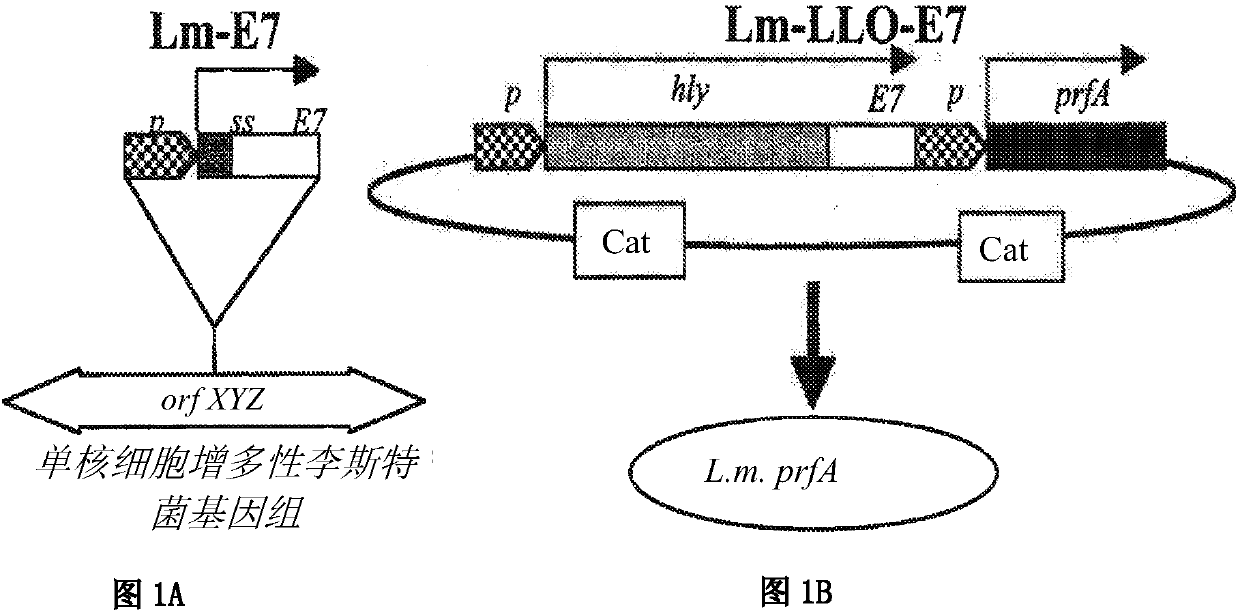
[1087] Listeria monocytogenes strains and reproduction
[1088] The Listeria strain used was Lm-LLO-E7, also referred ...
example 1
[1105] Example 1: LLO-antigen fusions induce anti-tumor immunity
[1106] result
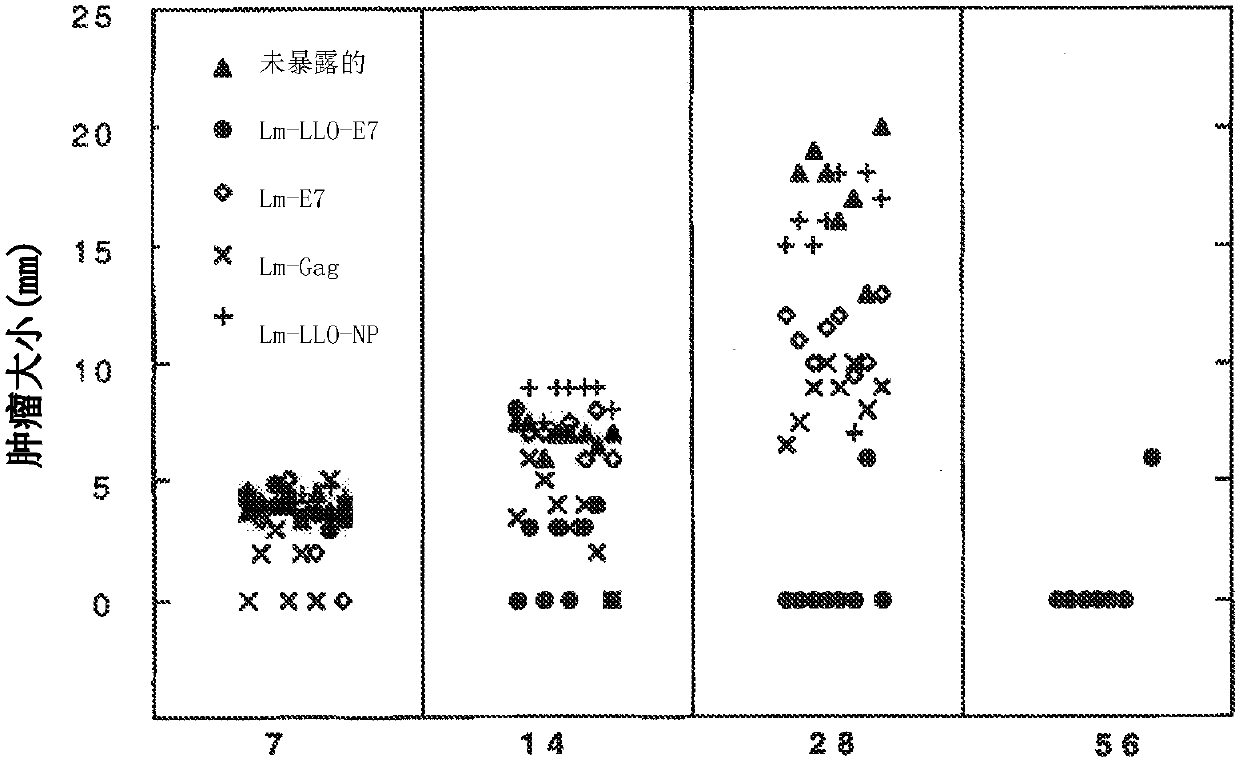
[1107] The ability of Lm-E7 and Lm-LLO-E7 to affect the growth of TC-1 was compared. Subcutaneous tumors were established on the left flank of C57BL / 6 mice. Seven days later, the tumor had reached a palpable size (4-5mm). On days 7 and 14, mice were inoculated with 0.1 LD 50 Lm-E7, Lm-LLO-E7 or Lm-Gag and Lm-LLO-NP as controls. Lm-LLO-E7 induced complete regression of 75% of established TC-1 tumors, while tumor growth was controlled in the other 2 mice in the group ( image 3 ). In contrast, immunization with Lm-E7 and Lm-Gag did not induce tumor regression. The experiment was repeated several times, always with very similar results. In addition, Lm-LLO-E7 also obtained similar results under different immunization schemes. In another experiment, a single immunization was able to cure established 5 mm TC-1 tumors in mice.
[1108] In other experiments, similar results were obtained with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com