Self-replicating vector for DNA immunication against HIV
A vector and recombinant vector technology, applied in the field of preparation of anti-HIV vaccine, treatment or prevention of HIV, can solve the problem that the virus cannot fully replicate due to the virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
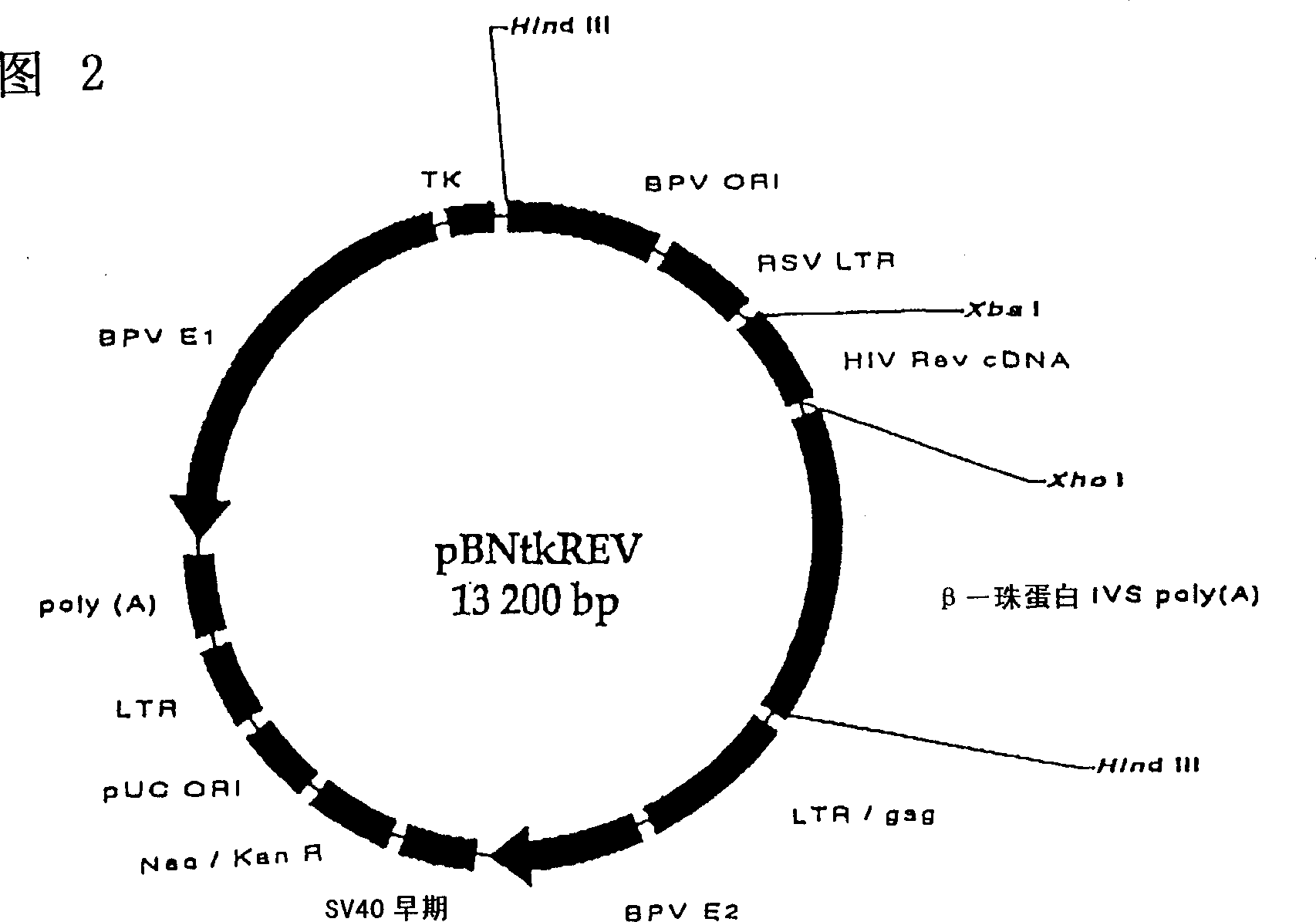
[0069] Example 1 Cloning of HIV-1 gene REV and TAT into self-replicating pBNsr-α and pBNtk plasmids to produce pBNtkREV and pBNsrαTAT first stage:
[0070] Isolated BRU ( HIV-1 REV and TAT genes in LAI (also known as LAI) (Wain-Hobson et al., Cell 40: 9-17, 1985): REV: 5'-TTTTTCTAGAACCATGGCAGGAAGAAGCGGA-3'5'-TTTTCTCGAGCTATTCTTTAGTTCCTGG-3'TAT: 5 '-TTTTTCTAGAACCATGGAGCCAGTAGATCCT-3'5'-TTTTCTCGAGCTAATCGAACGGATCTGC-3'
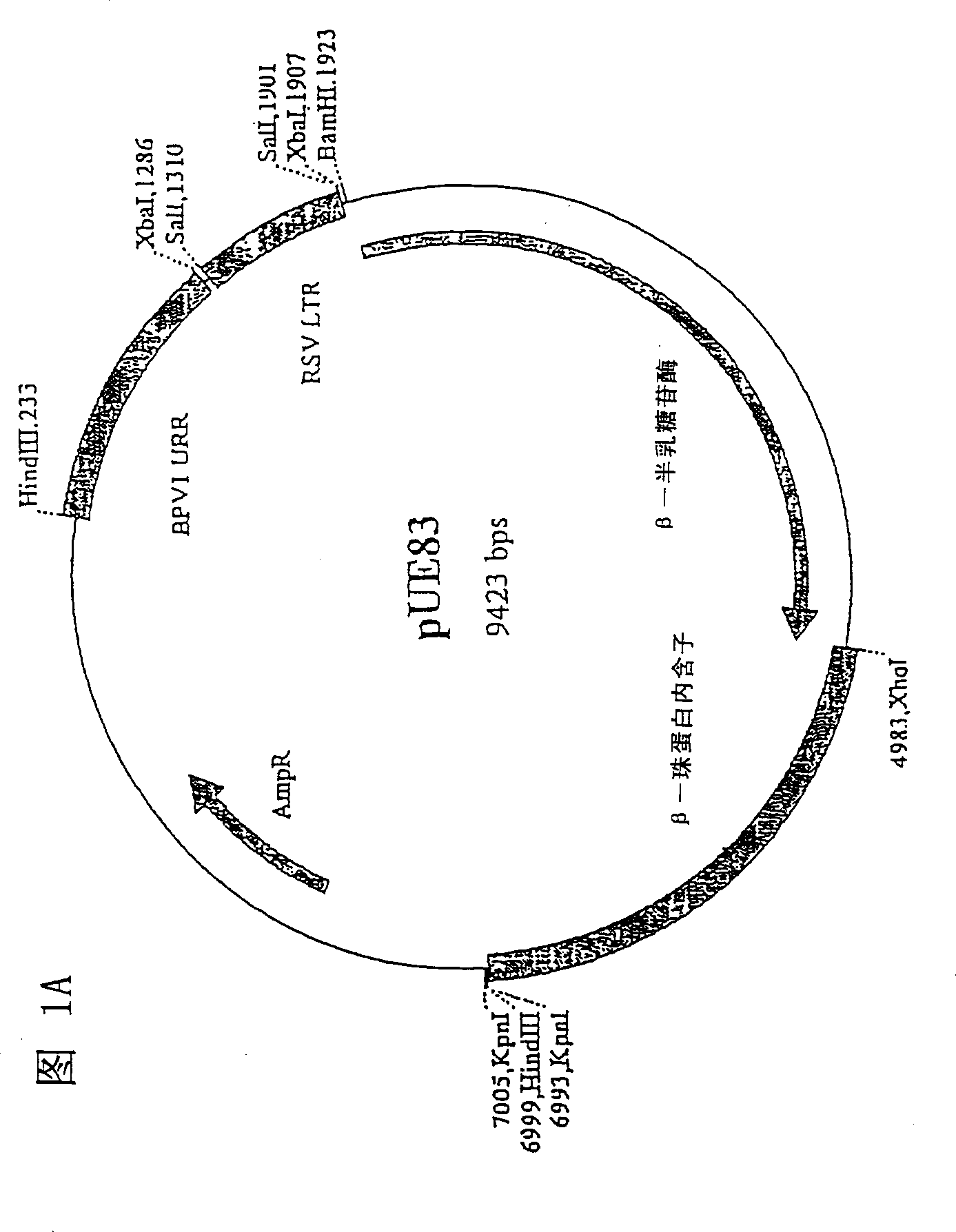
[0071] The amplified gene and the pUE83 shuttle vector (Fig. 1) were digested with XbaI and XhoI (New England BioLabs, USA) overnight at 37°C to obtain compatible ends. The digested DNA fragments were analyzed on a 1.5% agarose gel and further purified using a Band Prep kit (PharmaciaBiotech, Sweden). Incubate overnight at 16°C to ligate each gene into the vector with T4 DNA ligase (NewEngland BioLabs, USA). The competent E. coli cells in the One Shot kit (Invitrogen, Netherlands) were transformed with the ligation product, and the transformed cells were plated...
Embodiment 2
[0074] Example 2 Cloning of HIV-1 NEF into a self-replicating pBNsrα plasmid produces the first stage of pBNsrαNEF:
[0075] The HIV-1 NEF gene was obtained from a plasmid pcNEF vector containing the NEF gene of an LAI isolate inserted into a pcTAT vector lacking the TAT gene. The 1.3 kb fragment obtained by digesting pcNEF with SpeI and HindIII can be used for further cloning of the NEF gene. In order to prevent the formation of HindⅢ sites again during ligation, after HindⅢ digestion, the fragments need to be treated with a mixture of Klenow enzyme and dATP, dCTP, dGTP nucleotides, and then digested with SpeI. The resulting fragments were separated by electrophoresis on a 1% agarose gel with markers of standard size. Bands of the correct size were excised and DNA was recovered using the Sephaglas Bandprep kit (Pharmacia Biotech) following the manufacturer's protocol.
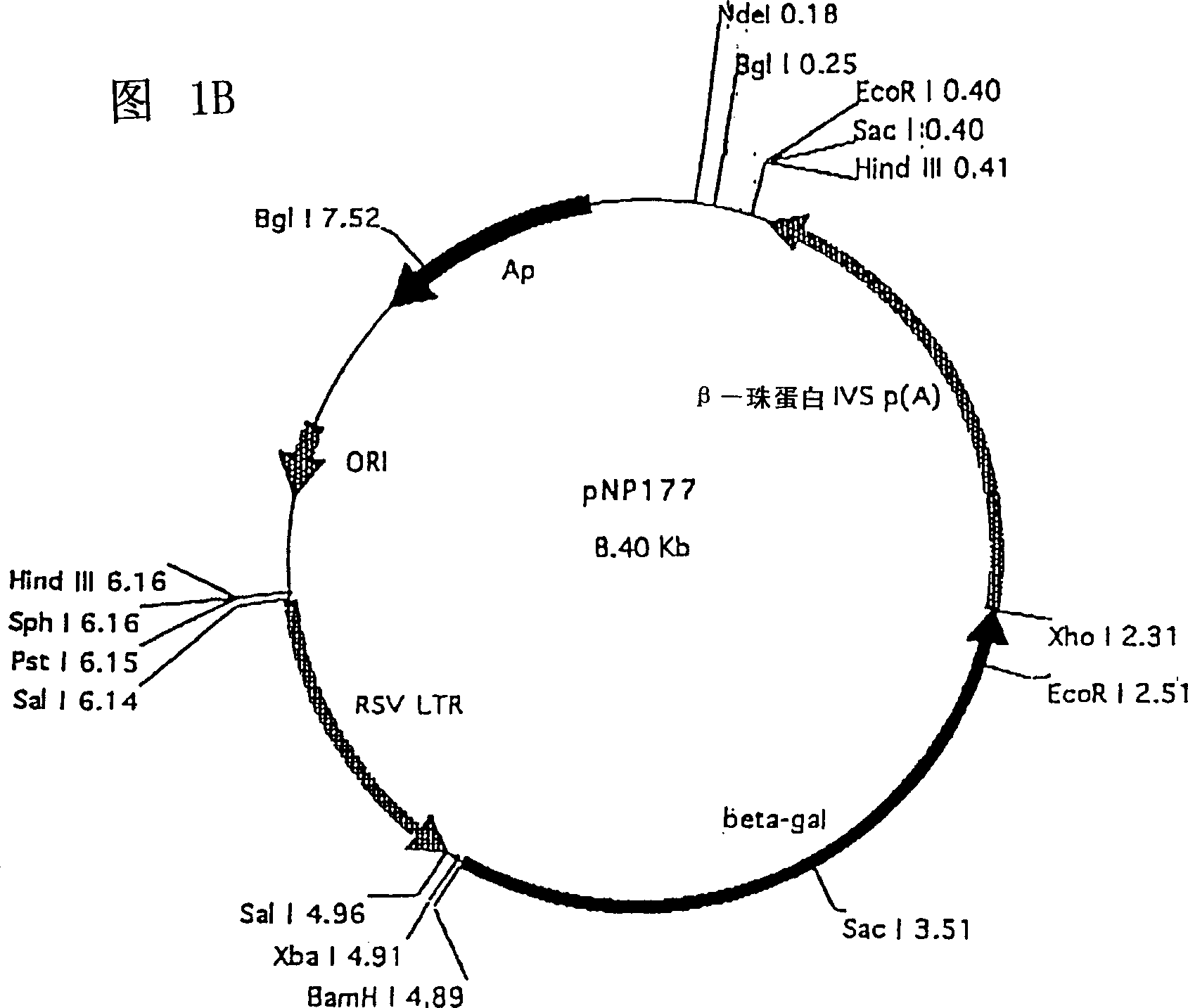
[0076] The shuttle vector pNP177 shown in Figure 1B was first digested with XhoI, then treated with Kleno...
Embodiment 3
[0079] Example 3 illustrates the in vitro expression of HIV-NEF 3A. transfection
[0080] To test the expression of the pBN-constructs in Examples 1 and 2, COS-7 cells were transfected with them by electroporation. 10 µg of pBNβ-Gal used as a control, and 10 µg of pBN-NEF co-transfected with 1 µg of pCMVβ-Gal were each electroporated into 3 million cells using salmon sperm vector DNA. Electroporation was performed at a capacitance of 960 mF and a voltage of 260 V, and protein concentration and β-Gal activity were measured to control transfection efficiency and to calibrate the amount of lysate in Western blot experiments. 3B. Immunohistochemistry and Western Blot
[0081] The harvested cells transfected with the pBN-construct were lysed for Western blotting, after lysis, the protein samples were boiled in sample buffer, electrophoresed in a 12% SDS polyacrylamide gel, and then transferred to a 5- % milk in TBS and blocked on a 2 μm nitrocellulose filter. The primary antib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com