Method for preparing recombinant cystatin C
A technology of cystatin and fusion protein, applied in the field of preparation of recombinant cystatin C, which can solve the problems of limited source, low yield and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] 1) Taking the human Cys C gene provided by NCBI as a reference, combined with the test design requirements of the present invention, the amino acid sequence shown in SEQ ID NO: 1 was determined without a tagged vector, after optimization of the synonymous codon preference of Escherichia coli ( SEQ ID NO: 2), the connection vector is pET-28a(+) (C-terminal fusion expression (His)6 tag in the vector), synthesized by Nanjing GenScript Biotechnology Co., Ltd.
[0089] The amino acid sequence of the tagged fusion Cys C protein is shown in SEQ ID NO: 3. In the fusion Cys C protein, the first 26 amino acids at the N-terminal of SEQ ID NO: 1 are excised to better promote the soluble expression of Cys C protein. After optimization of synonymous codon preference in Escherichia coli (SEQ ID NO:4), the connection vector was pET-32a(+) (the C-terminal fusion expression (His)6 tag in the vector), provided by Nanjing GenScript Biotechnology Co., Ltd. Ltd Synthetics.
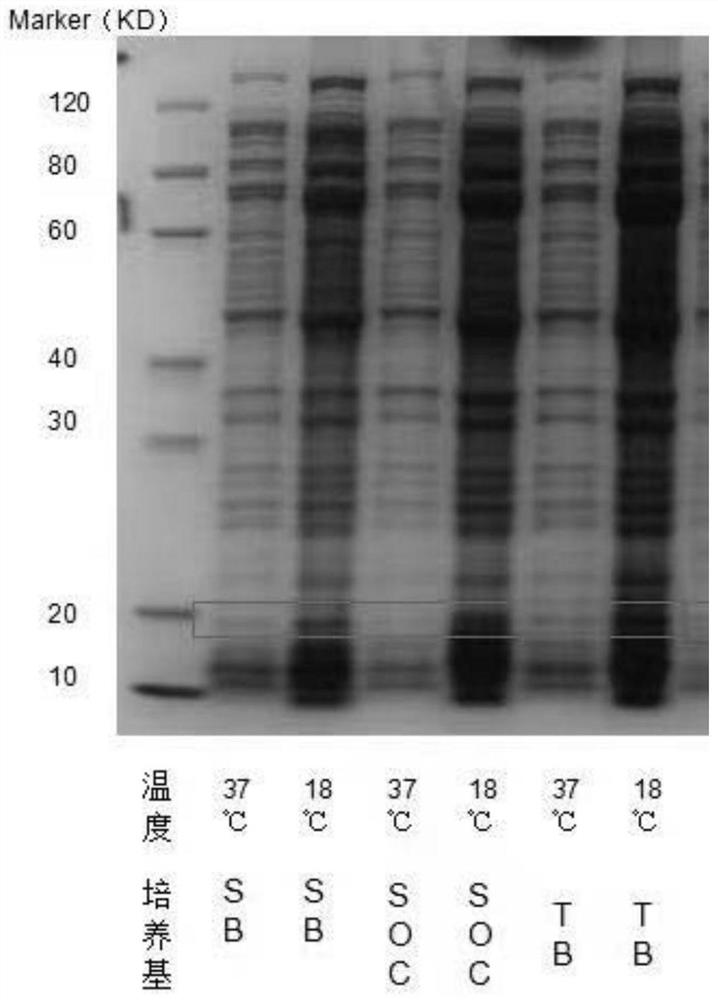
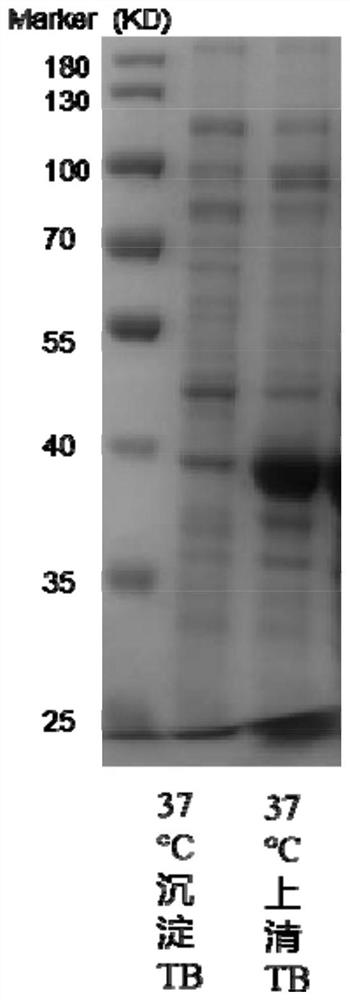
[0090] 2) The r...
Embodiment 2
[0098] Example 2 Purification of a large amount of expression products
[0099] Cultivate 1.5L of bacterial liquid in a shake flask, and the wet weight of bacterial cells collected by centrifugation is 26g. Weigh about 4g of bacteria, add 35ml LysisBuffer and resuspend on ice. After sonication, centrifuge at 20,000 rpm at 4°C for 30 min, take the supernatant, and filter it with a needle filter of 0.22 μm to obtain the filtered bacterial liquid.
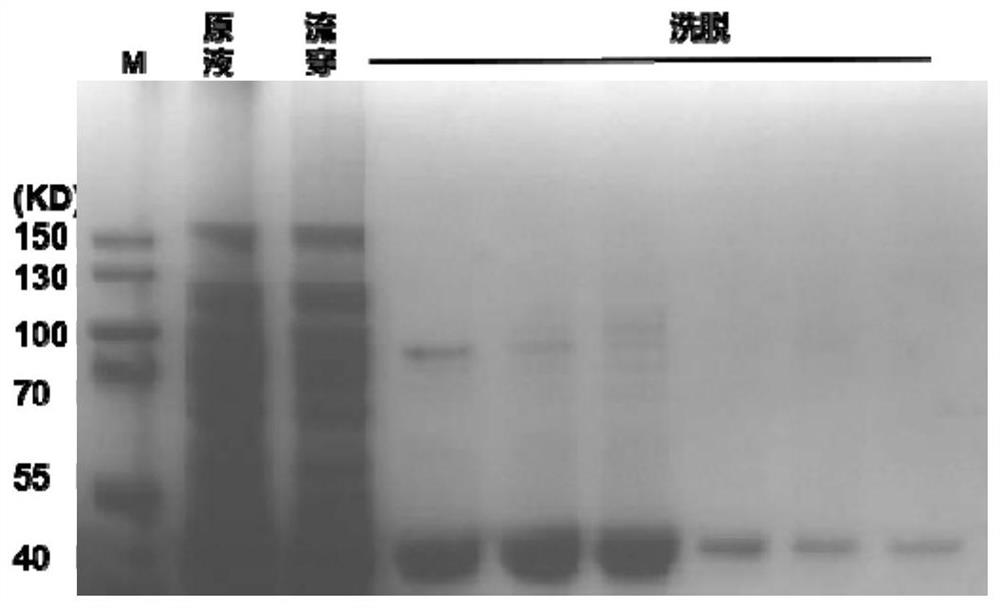
[0100] After filtering, pass through Ni-column affinity chromatography, and the protein eluted with 50mM Tris-HCl, 50mM NaCl, 200mM imidazole pH7.0 is the target protein, and 7ml of protein is obtained by elution with a concentration of 2.13mg / ml. The electrophoresis diagram is as follows image 3 shown.
[0101] The calculated target protein expression content is 97mg / L, and the purity can reach 95%.
Embodiment 3
[0102] Embodiment 3 protein stability test
[0103] The obtained target protein was divided into 2mL EP tubes, 1mL / tube, and sealed with parafilm.
[0104] There are 3 tubes in each batch, one of which is placed at 4°C as a control, and the remaining 4 tubes are placed at 37°C for an accelerated one-week test.
[0105] Samples were taken for identification on 3 and 7 days respectively, and the stability of the protein was tested by UNcle (Unchained Lab, United States, UNcle multifunctional protein stability analysis system).
[0106] Figure 4 The results showed that the stability of the recombinant fusion Cys C protein was better after being placed at 37°C for 3 and 7 days, and there was no significant difference with that at 4°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com