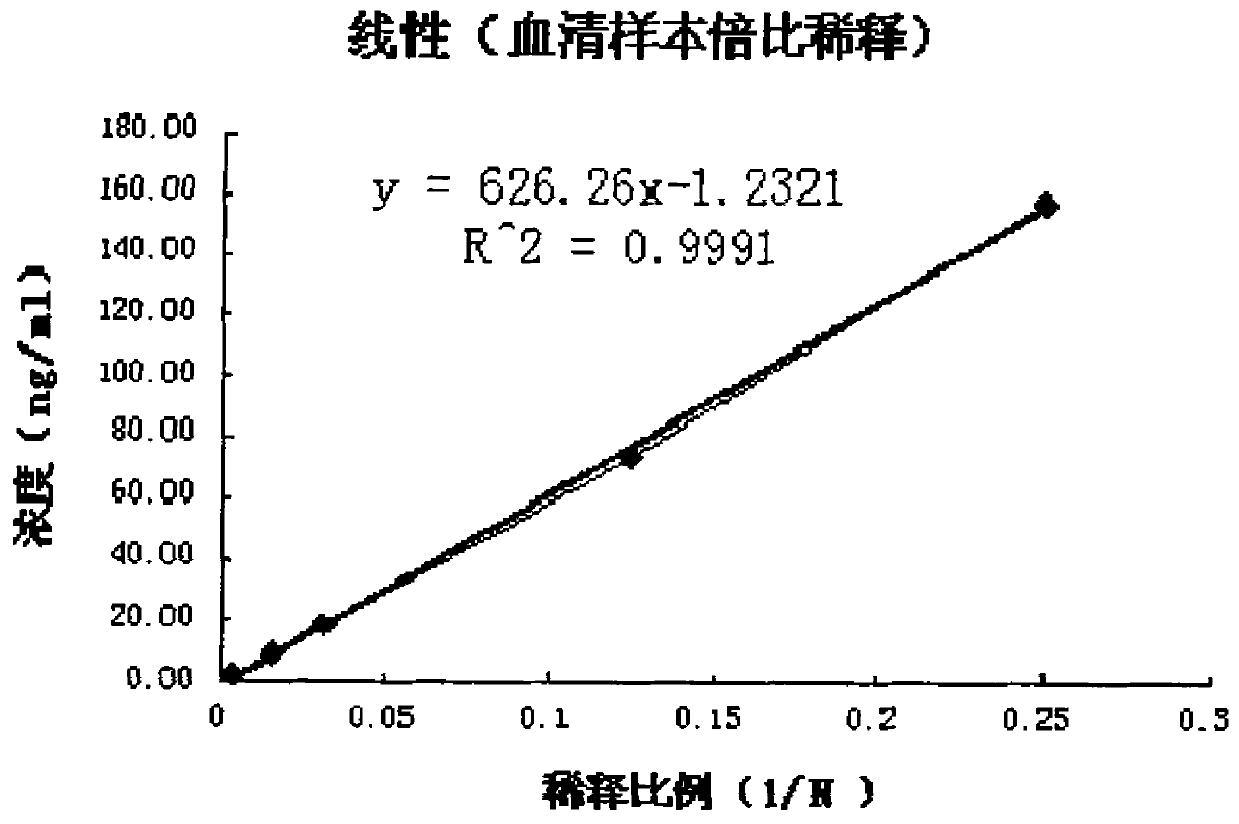
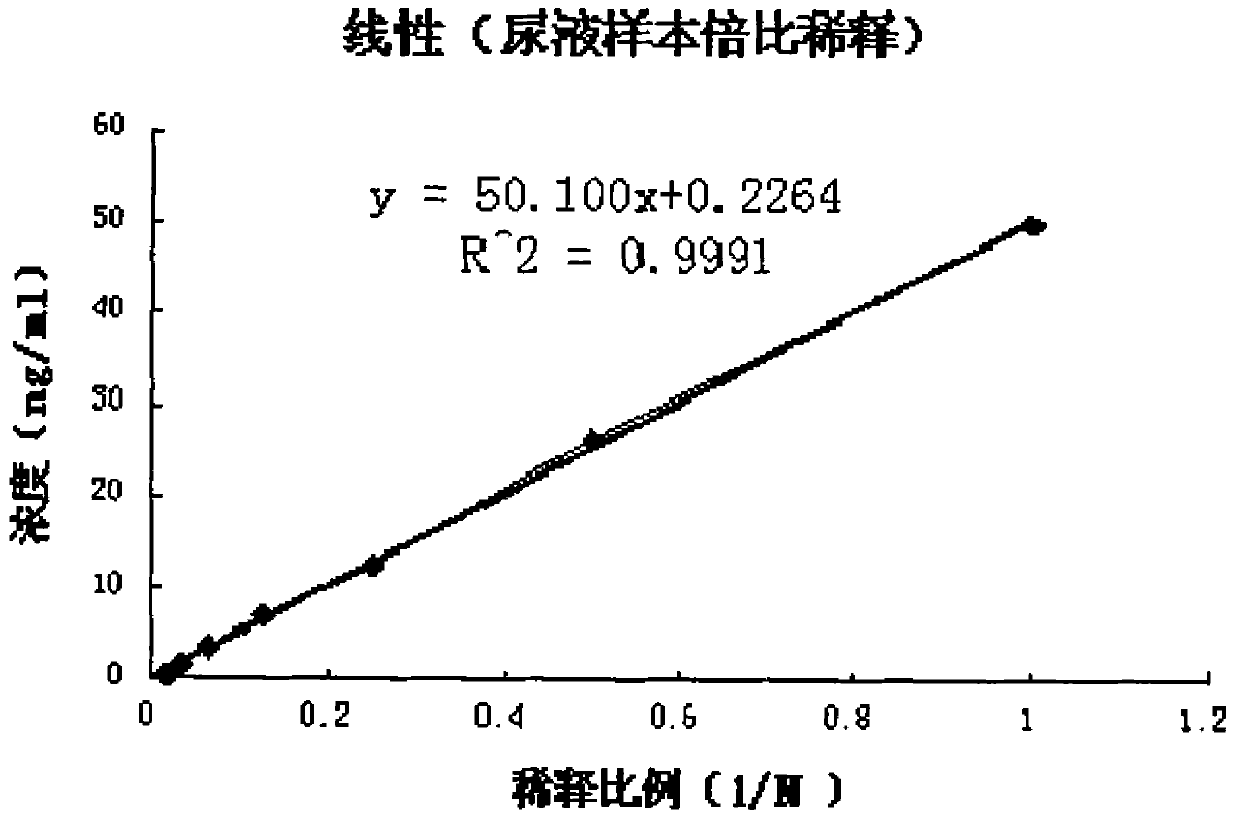
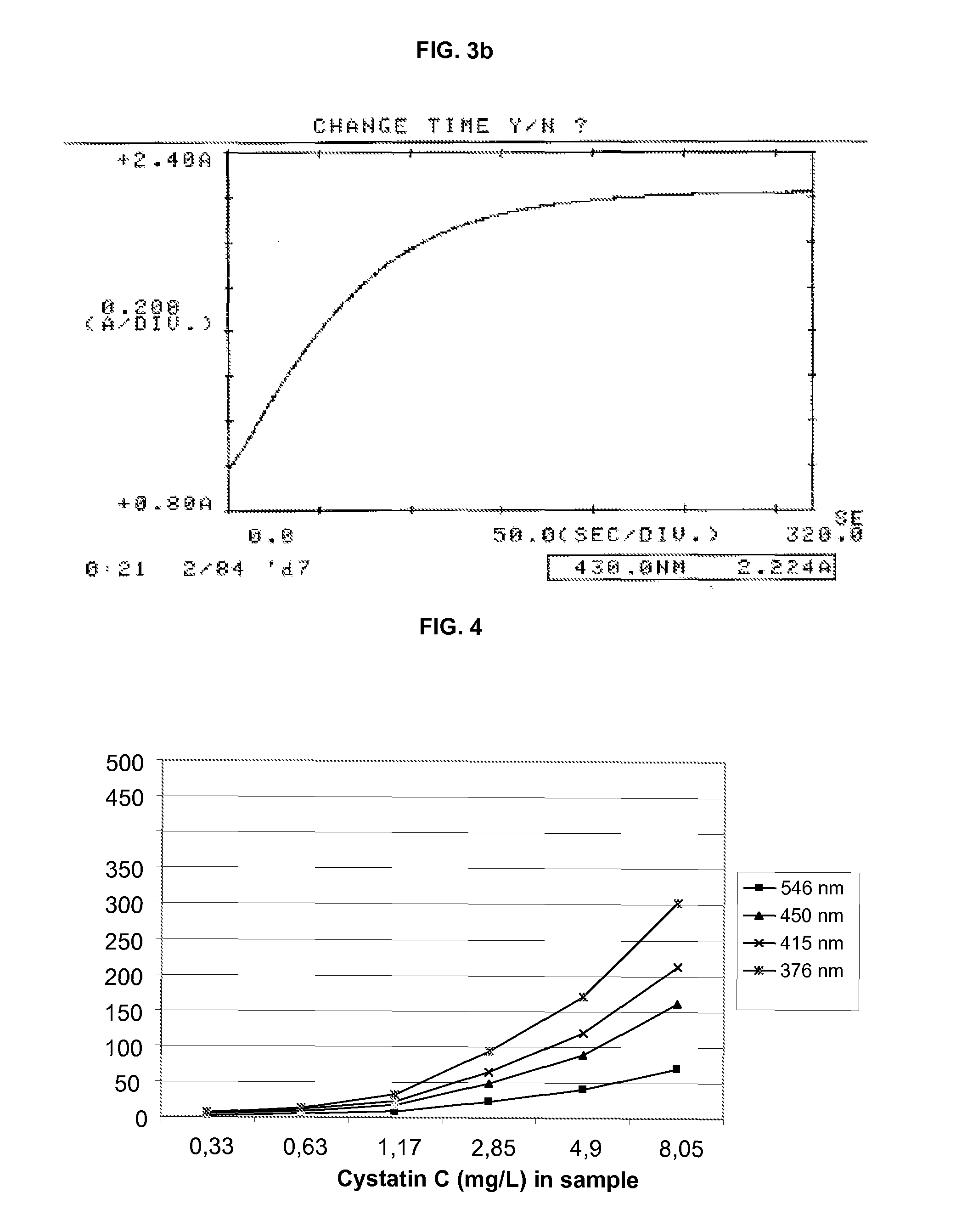
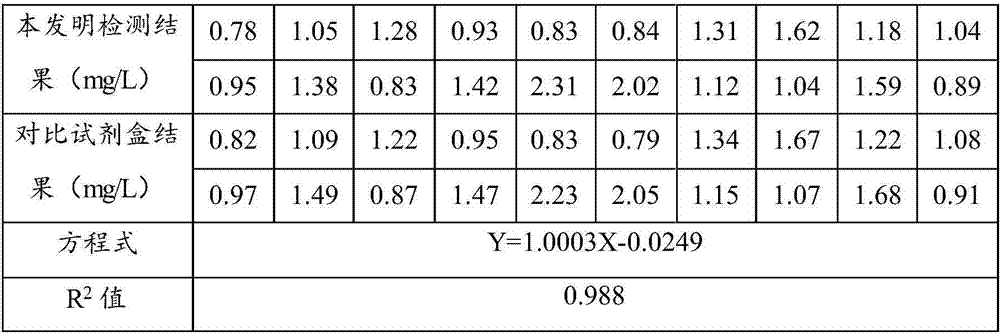
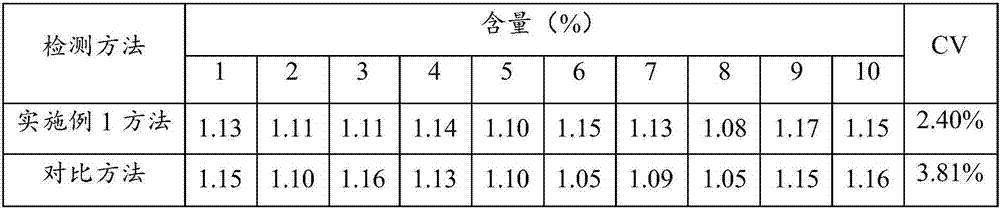
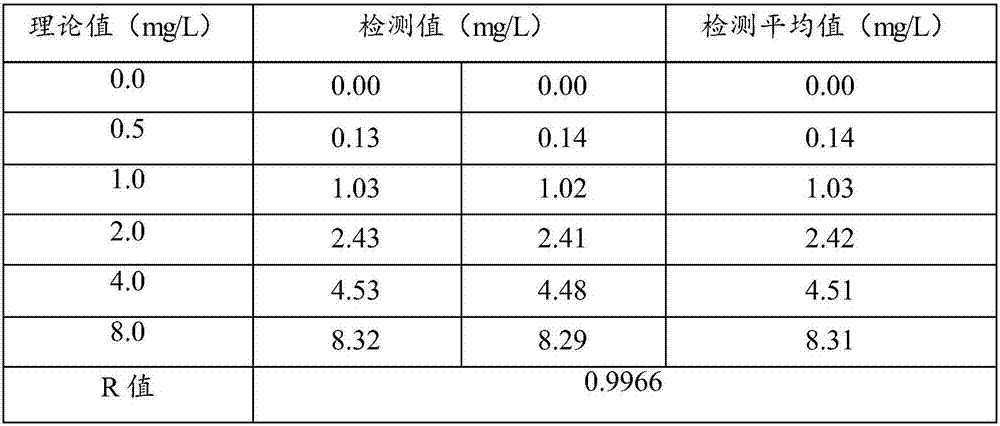
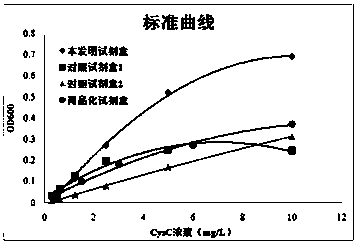
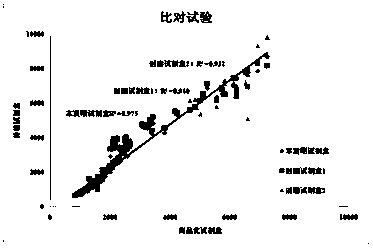
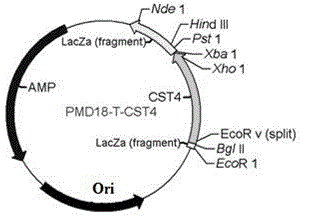
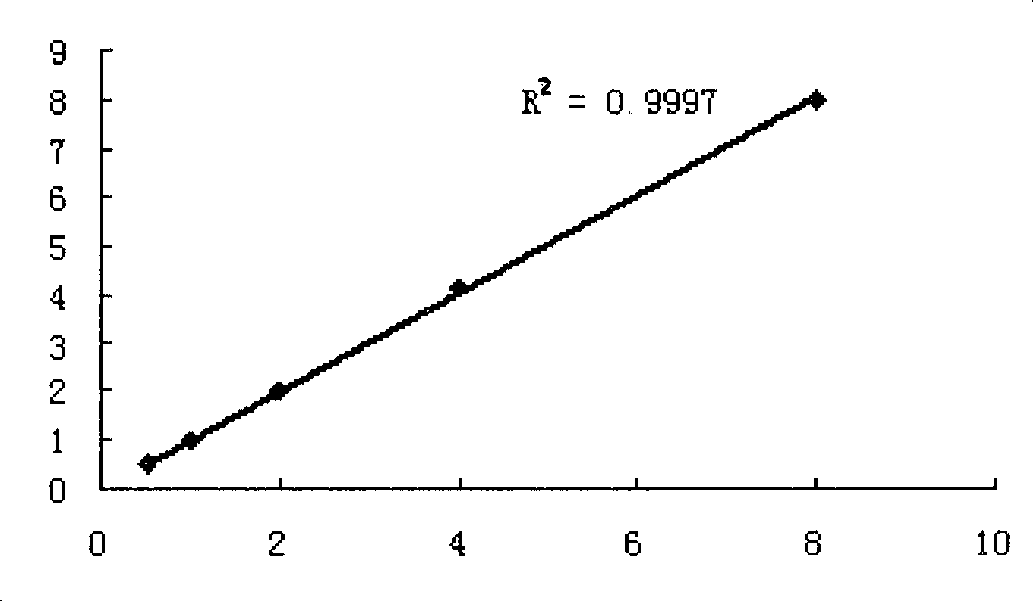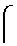Patents
Literature
69 results about "Cystatin S" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
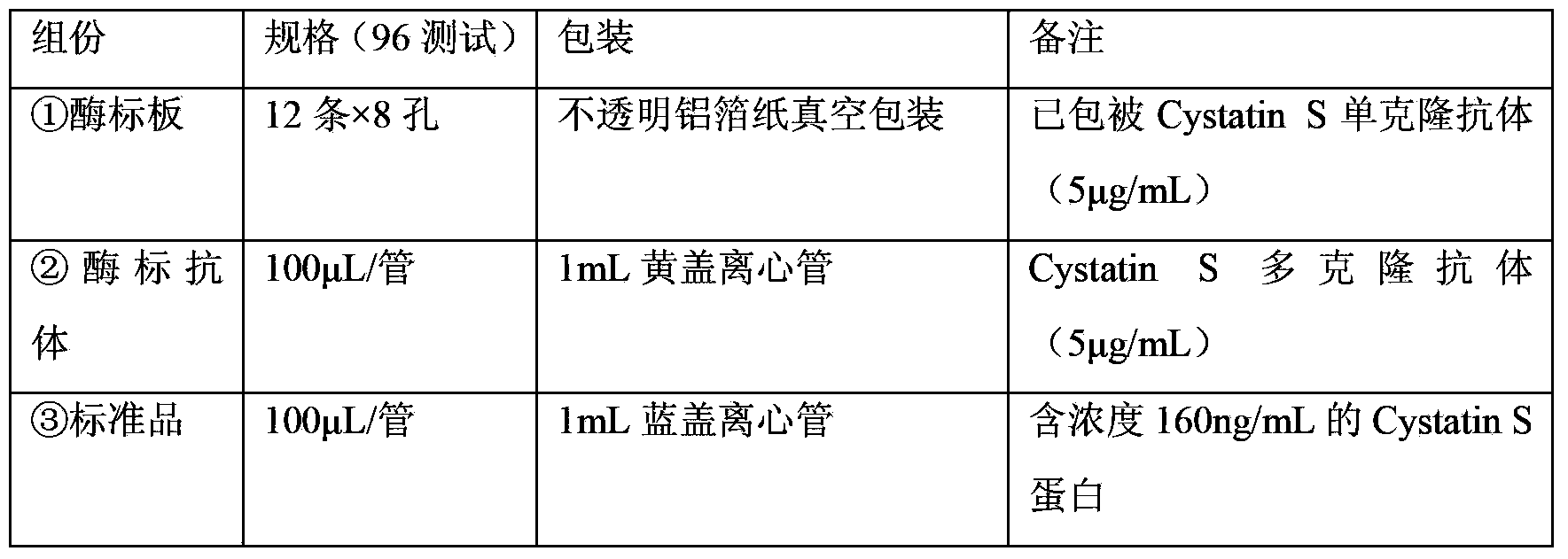
Magnetic particle separation chemiluminescence immunoassay method of human cystatin C
InactiveCN101937000AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceBiological testingFiltrationBlood plasma
The invention provides an in-vitro detection method of human cystatin C. The method adopts a magnetic particle separation chemiluminescence immunoassay technology which is a product integrating an enzyme labeling technology, a magnetic particle separation technology and a chemiluminescence detection technology and has the advantages of high sensitivity, good specificity, good repetitiveness, and the like. The method can be used for measuring the content of cystatin C in the serum, the plasma and the urine of a person, and the indexes are mainly used for clinically evaluating the renal function and mainly used for monitoring the filtration rate of glomerulus, the renal tubular dysfunction and various secondary nephropathies.
Owner:北京倍爱康生物技术有限公司
Recombinant human cystatin C genes, and expression and use thereof
InactiveCN101413001ASolve bottlenecksBacteriaImmunoglobulins against animals/humansMonoclonal antibodyCystatin C Gene
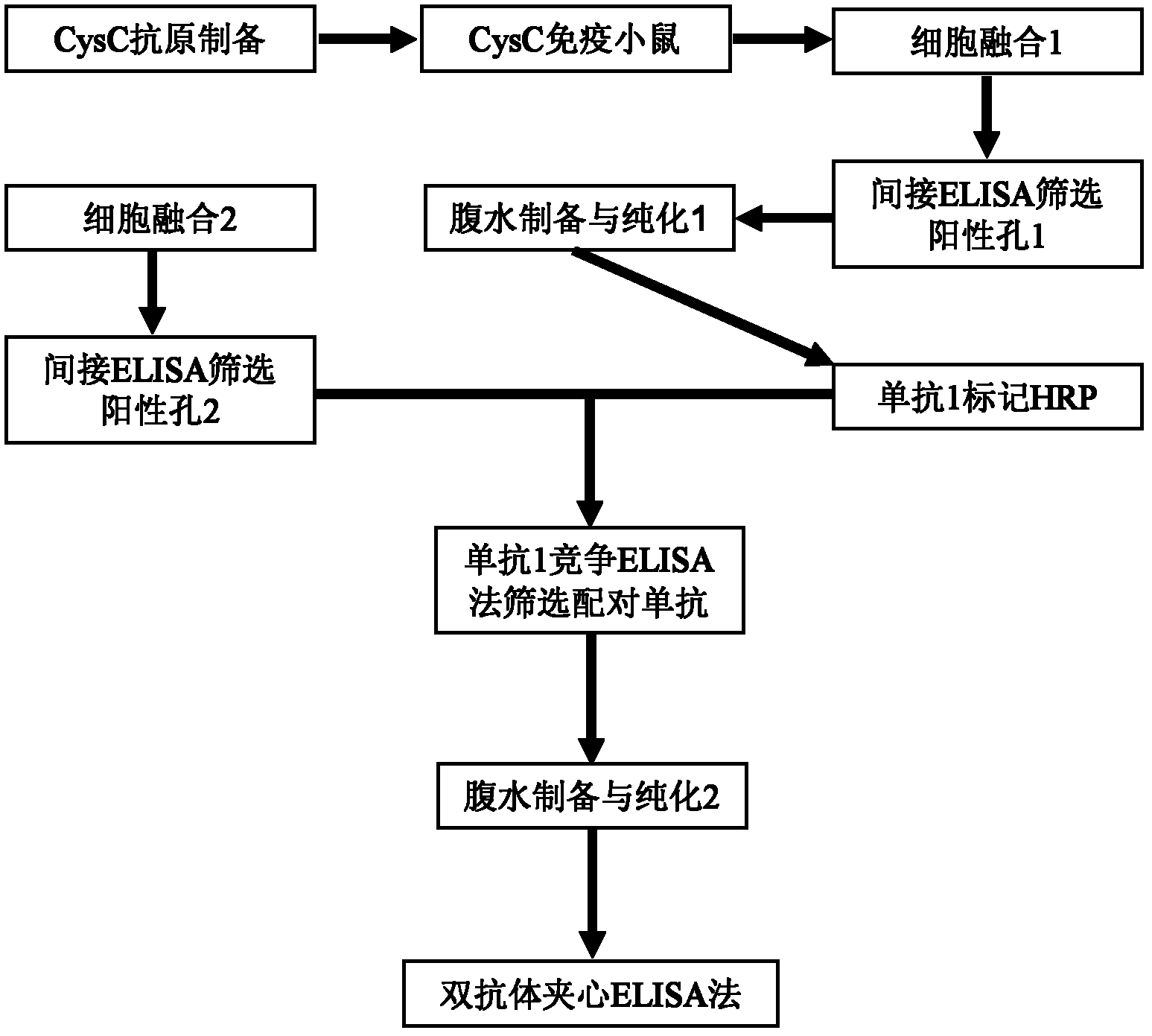
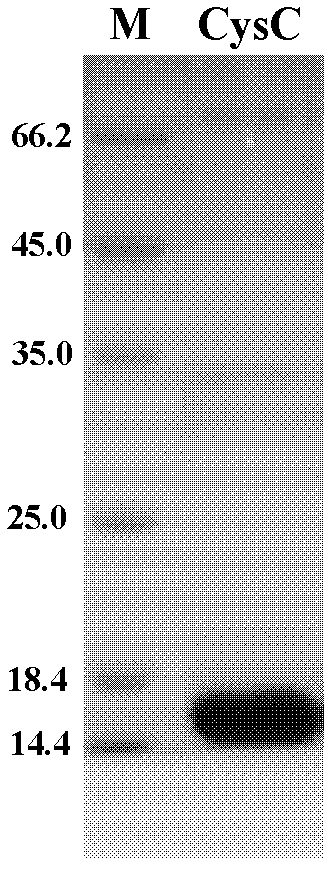
The invention discloses a recombinant human cystatin C gene and expression and application thereof. A truncated human cystatin C gene has a nucleic acid sequence as shown as in SEQ ID NO:1; the truncated human cystatin C protein has an amino acid sequence as shown in SEQ ID NO:2. The invention uses a genetic engineering technique to express and purify a great deal of stable recombinant soluble cystatin C protein and obtains a pair of monoclonal antibodies using the protein to prepare stable cystatin C diagnostic reagents and quality control products thereof, thereby solving bottleneck problems of independent research and development of a cystatin testing reagent box.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Method for preparing CysC-paired monoclonal antibody
ActiveCN102321176AReduce workloadExpand the scope of the filterImmunoglobulins against protease inhibitorsAntigenPeroxidase
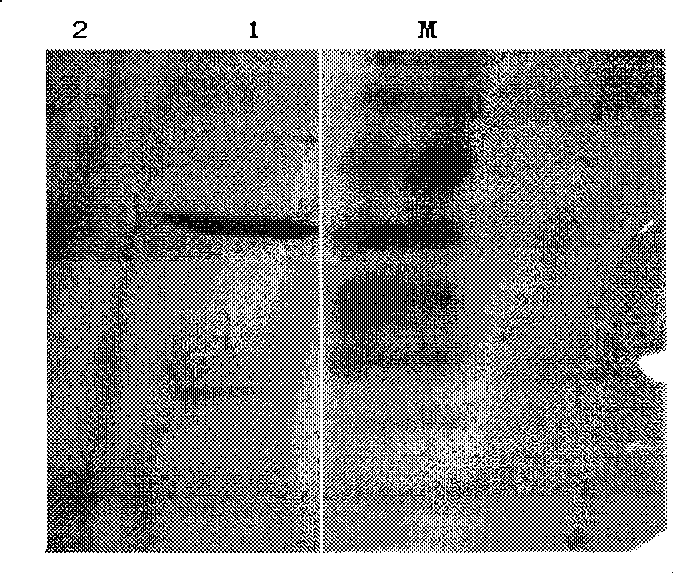
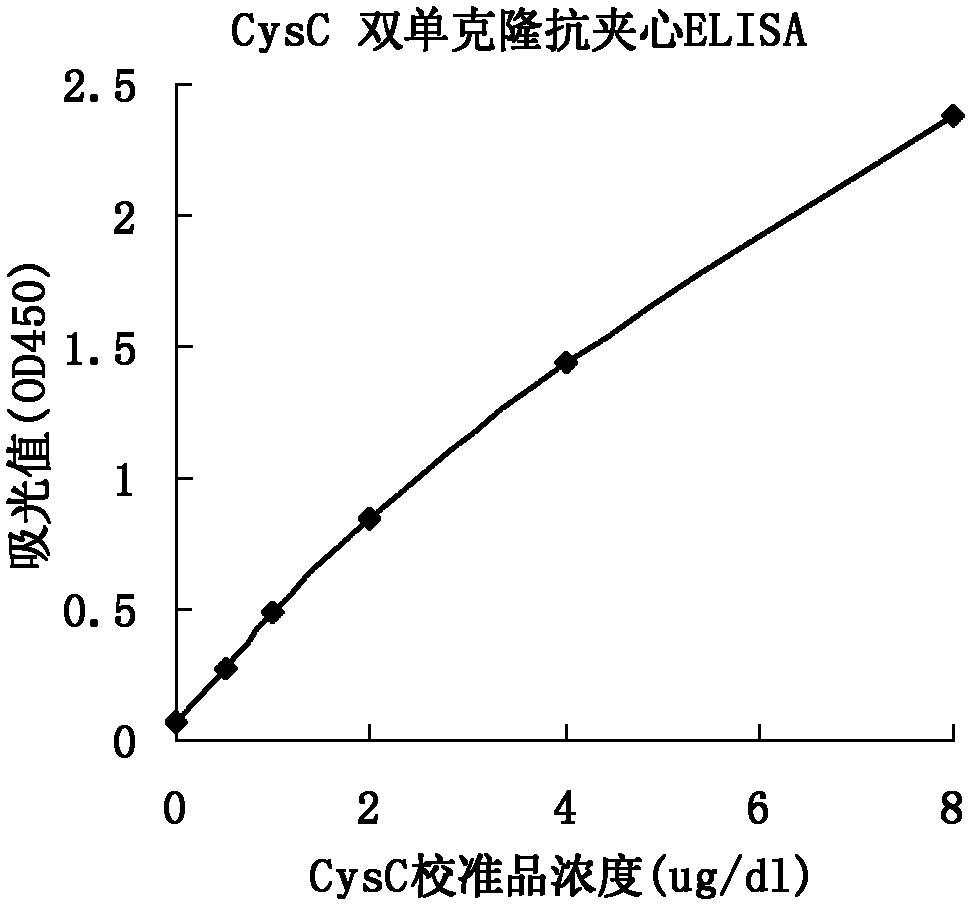
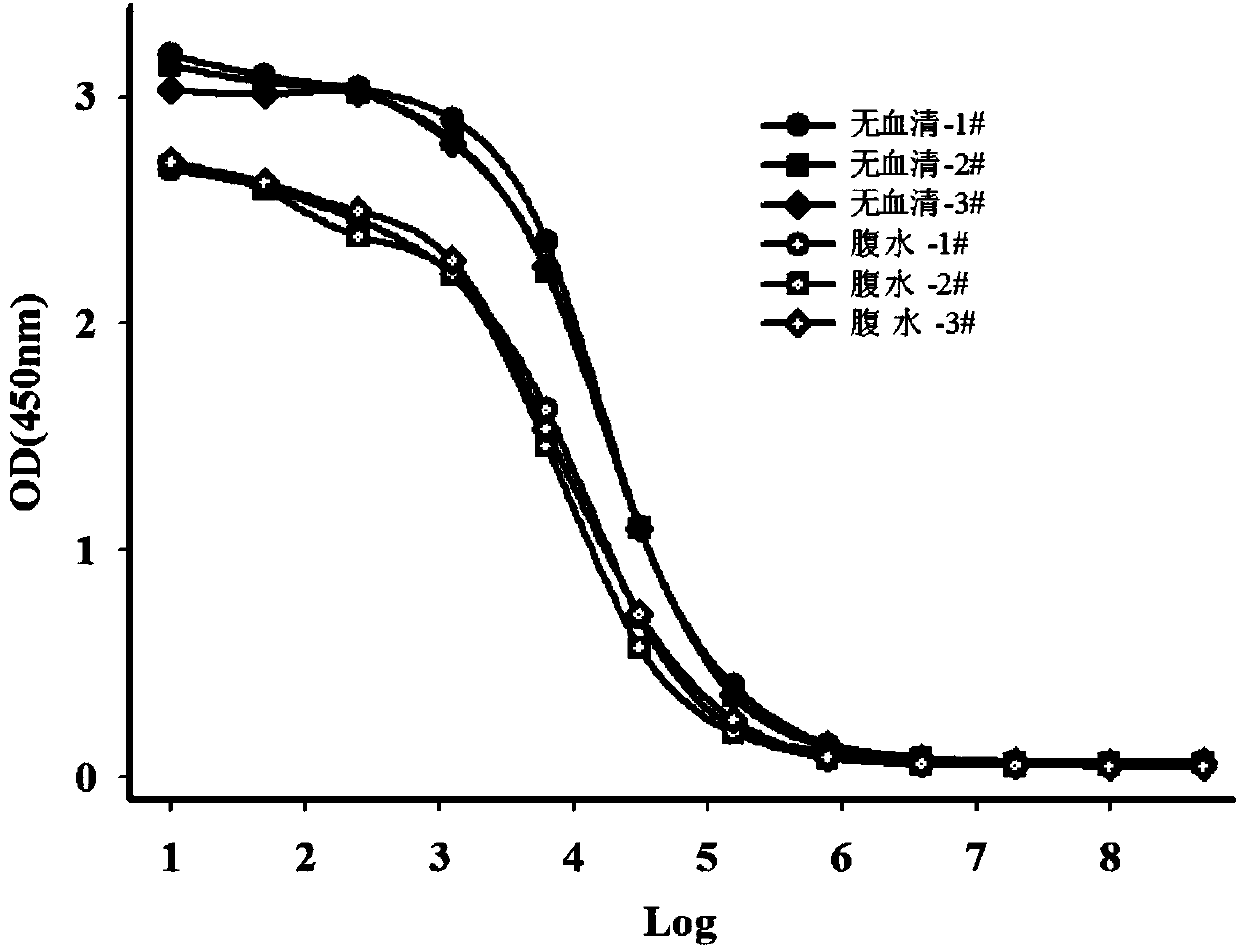
The invention relates to the field of protein engineering, in particular to a method for preparing CysC-paired monoclonal antibodies, which comprises the following steps of: screening hybridoma cells having specific binding with CysC after fusing mouse splenic cells and myeloma cells capable of generating CysC antibodies, preparing cells for generating the monoclonal antibodies, finishing first-time cell fusion, further purifying out the monoclonal antibodies, and selecting one monoclonal antibody to be marked with horse radish peroxidase; then implementing the second-time fusion of the mouse splenic cells and the myeloma cells; finishing CysC-antigen coating on an elisa plate, adding a hybridoma-cell cultural supernatant obtained through the second-time cell fusion to elisa-plate holes, arranging a blank control hole, subsequently adding the monoclonal antibody marked with the horse radish peroxidase to the elisa-plate holes, incubating at the temperature of 37 DEG C for 30 minutes, adding a substrate to each hole for color rendering, and measuring a light absorption value under 450nm after stopping reaction through sulfuric acid; and selecting the uninhibited holes as the paired monoclonal antibodies.
Owner:BEIJING LEADMAN BIOCHEM
Turbidimetric immunoassay for assessing human cystatin c
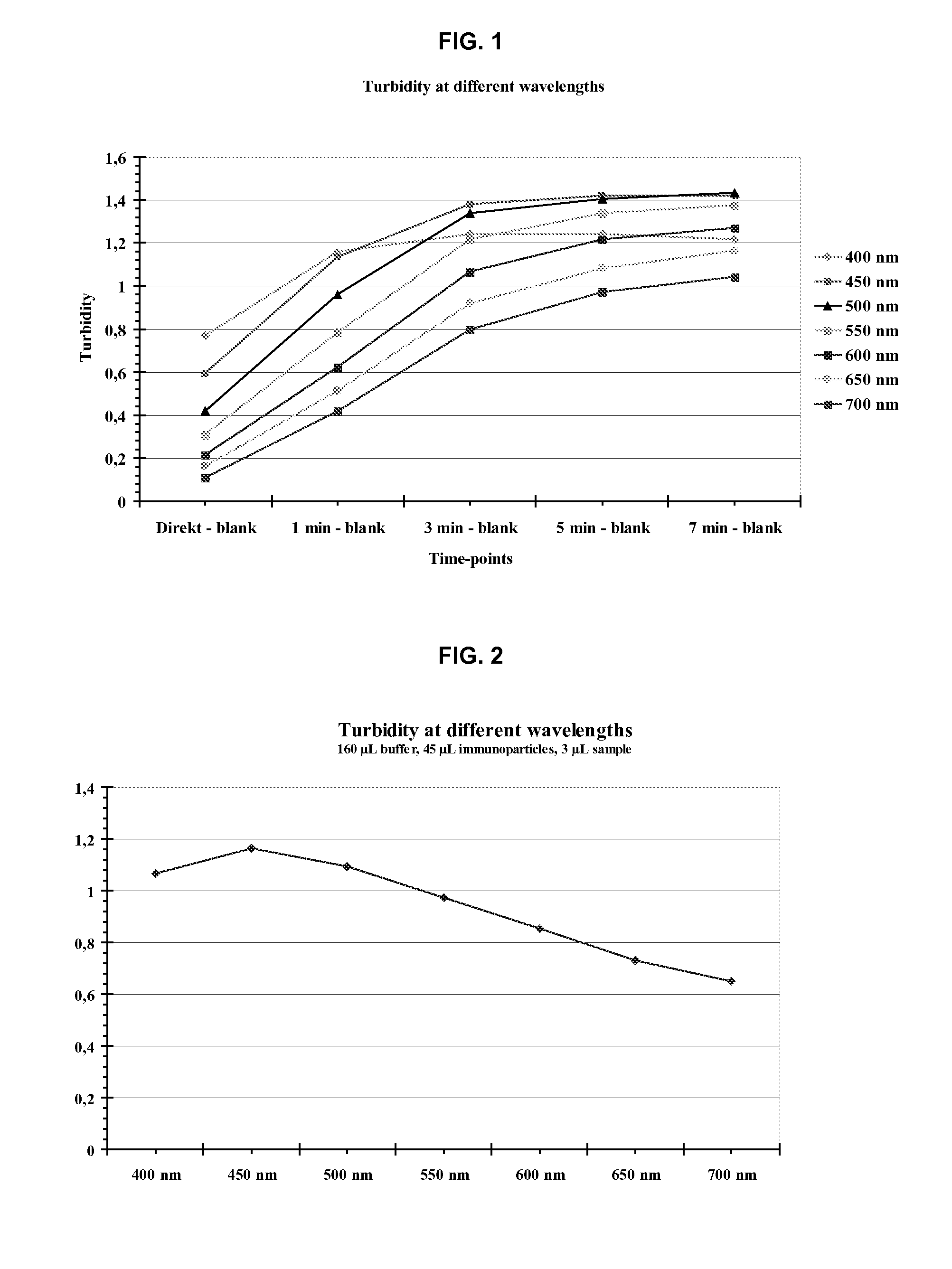
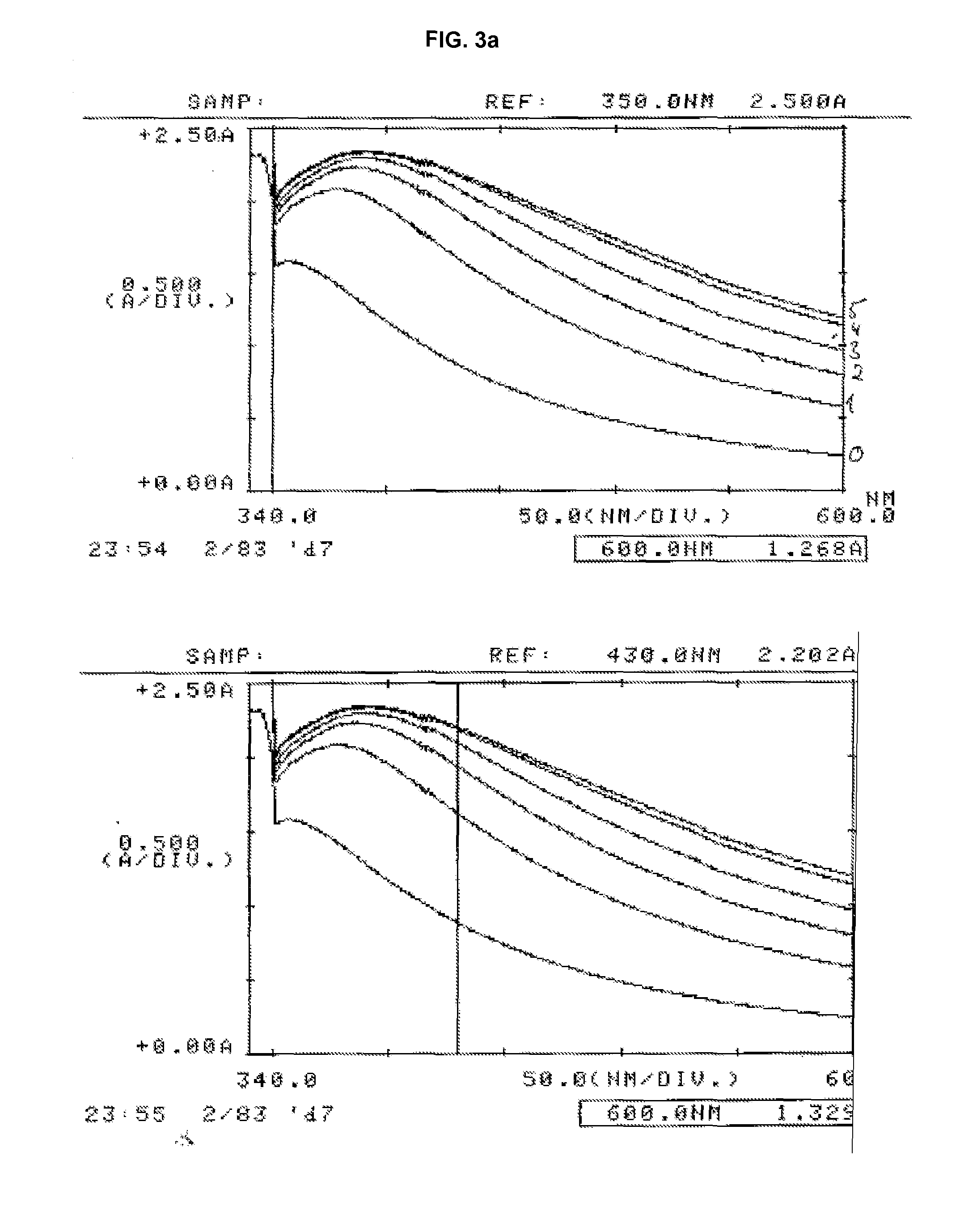
ActiveUS20100047922A1Strong and faster signalReduce distractionsDisease diagnosisImmunoglobulinsTurbidityBody fluid
There is a demand for improved turbidimetric immunoassays for human Cystatin C in biological samples, especially in human clinical samples of body fluids. The present invention provides a turbidimetric immunoassay method and reagent set enabling measurement of human Cystatin C by turbidimetric methods, resulting in a surprisingly stronger and faster turbidimetric signal than in the present state of the art. The increased and faster signal is accomplished by the use of new reagents and compositions, and enables shorter assay times and kinetic reading with a stronger signal, improving overall assay speed and quality. Improved robustness to lipid interference and improved linearity is achieved.
Owner:GENTIAN AS
Cystatin C detection kit and preparation method therefor
ActiveCN103645323AHigh sensitivityGood precisionBiological material analysisBiological testingMedicinePolystyrene
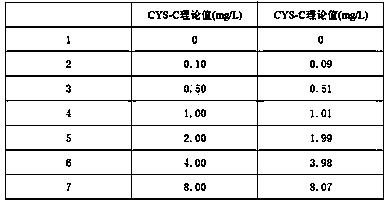
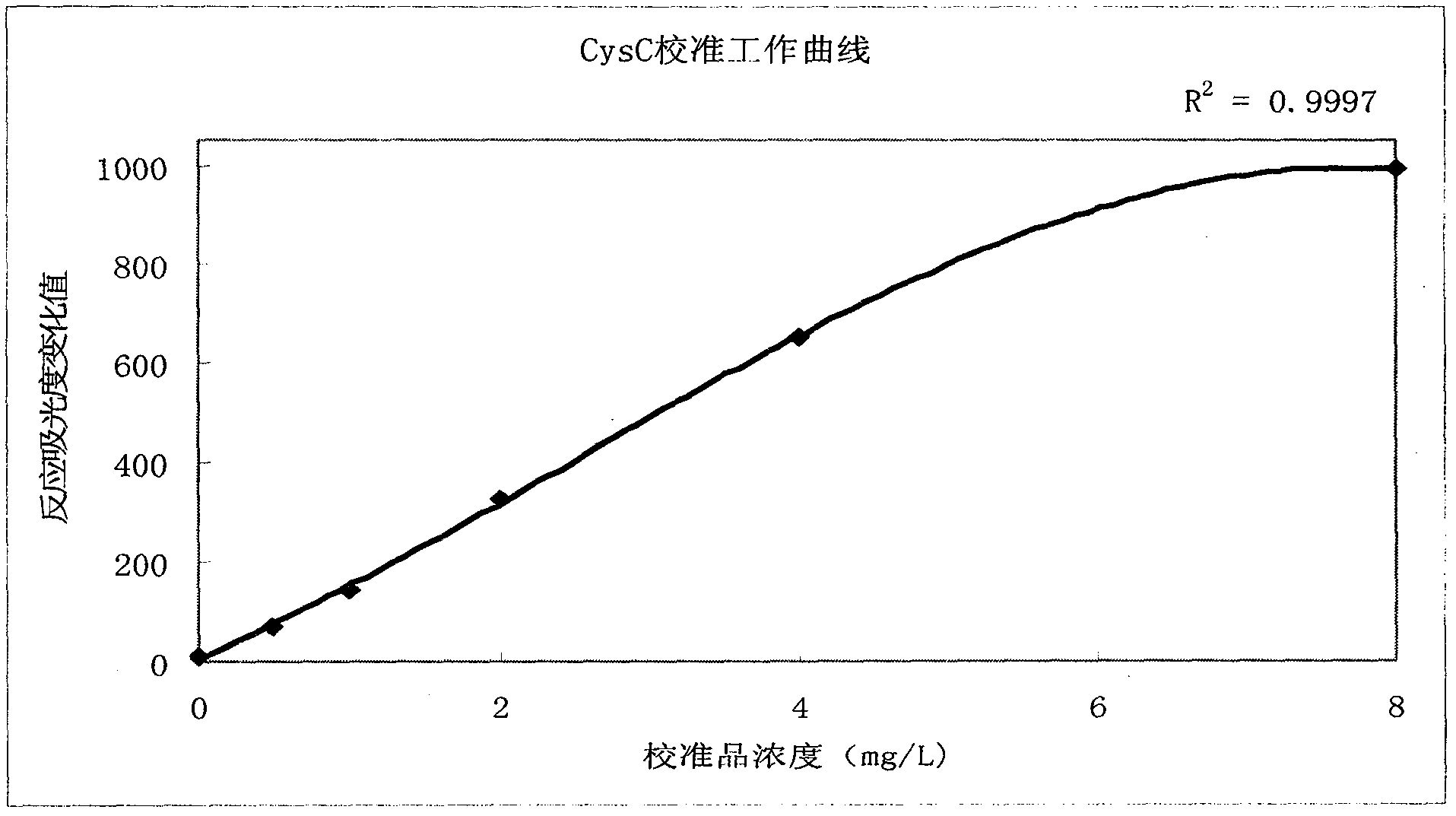
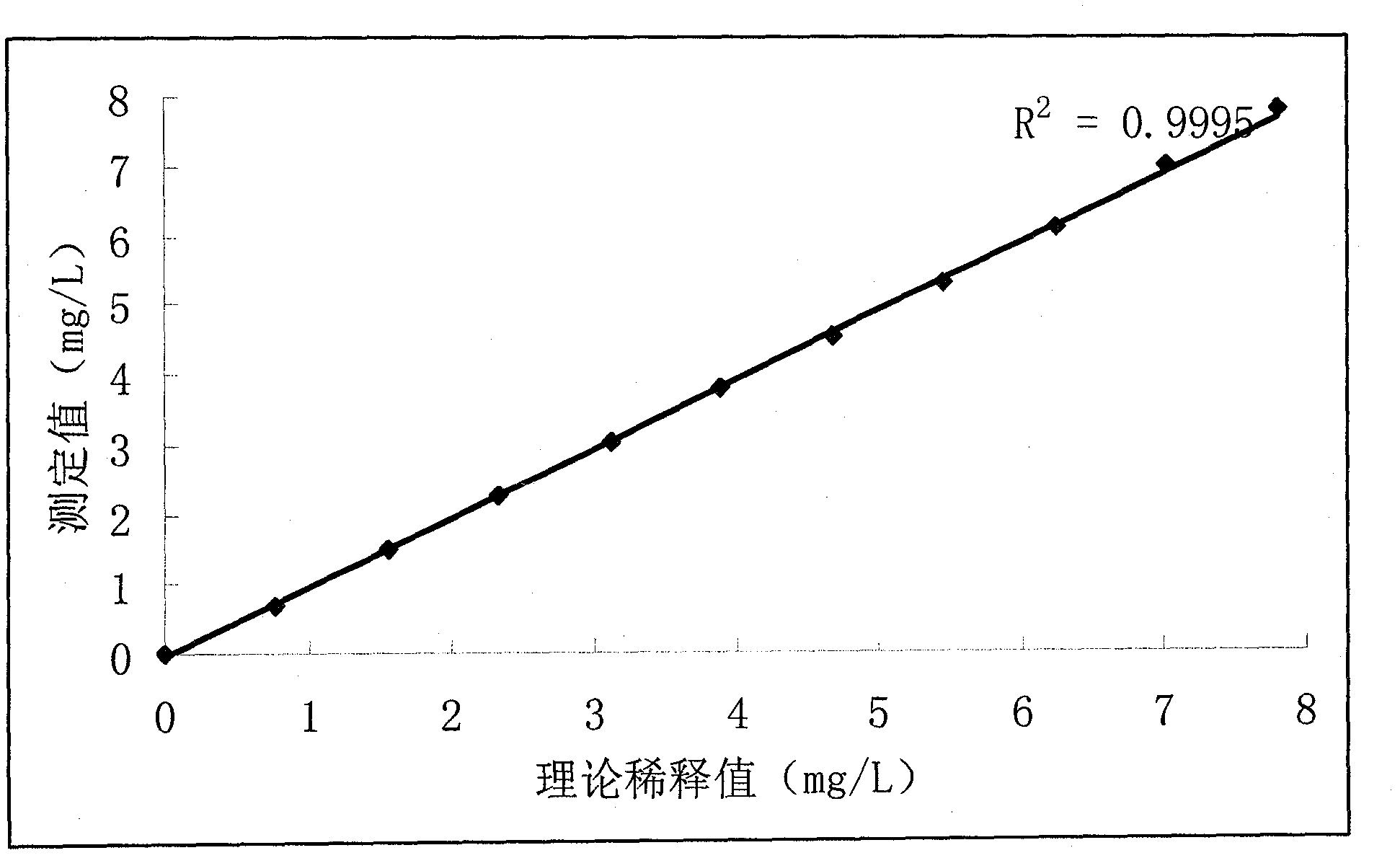
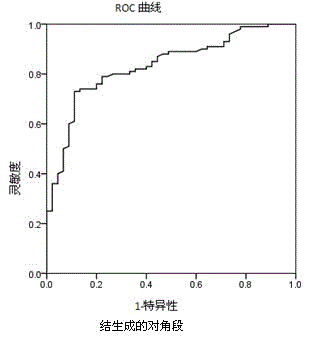
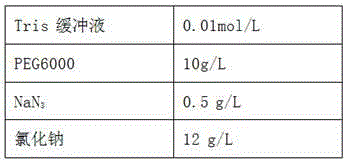
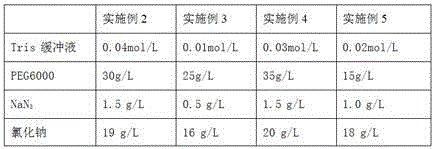
The invention relates to the field of medical immunology in vitro diagnosis, and especially provides a detection kit for detecting cystatin C in serum. The detection kit comprises a reagent R1, a reagent R2 and calibration materials. The ingredients of the reagent R1 are a Tris buffer with a concentration of 0.01-0.05mol / L, PEG 6000 with a concentration of 10-40g / L, NaN3 with a concentration of 0.5-1.5g / L, and sodium chloride with a concentration of12-20g / L, and the pH value is 8.0-8.5. The ingredients of the reagent R2 are a Tris buffer with a concentration of 0.01-0.02mol / L, and polystyrene latex granules coated by goat-anti-human cystatin C polyclonal antibodies with a concentration of 0.5-3.0 g / L. The calibration materials are six gradient standards with cystatin C and the contents of cystatin C are 0.1, 0.5, 1.0, 2.0, 4.0 and 8.0mg / L. The solution is a Tris buffer with a concentration of 0.01mol / L. The kit has simple composition, good stability and high accuracy.
Owner:浙江夸克生物科技有限公司
Recombined human cystatin-C protein with natural activity and preparation method thereof
ActiveCN103014047AImprove solubilitySolve the key problems of independent research and developmentMicroorganism based processesProtease inhibitorsBiotechnologyProteinase activity
The invention discloses a recombined human cystatin-C protein with natural activity and a preparation method thereof. According to the preparation method, lots of stable recombined human cystatin-C proteins are expressed and purified by employing a gene engineering technique, and the recombined human cystatin-C proteins are cut by utilizing the protease, so that the recombined human cystatin-C protein has high natural activity. The recombined human cystatin-C protein can be used for immune preparation of monoclonal antibodies and multi-antibody blood serum, and excellent raw materials are provided for preparing stable cystatin-C detection agents and quality control products thereof.
Owner:深圳前海菲鹏基因科技有限公司 +1
Fluorescent probe for detection of cystatin C and construction method thereof
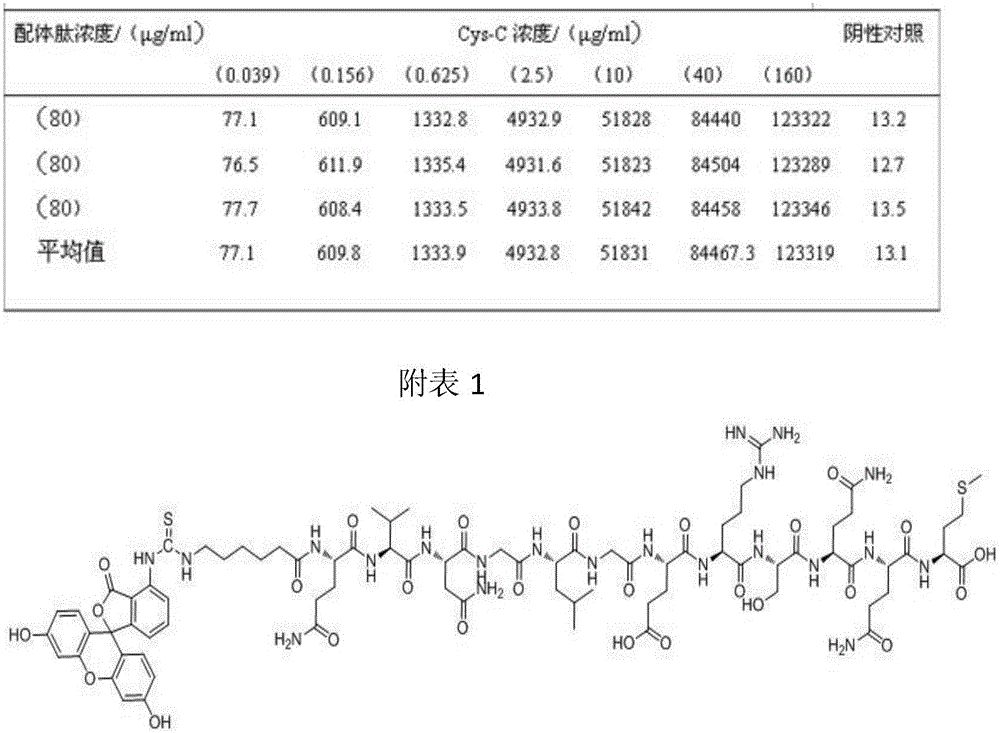
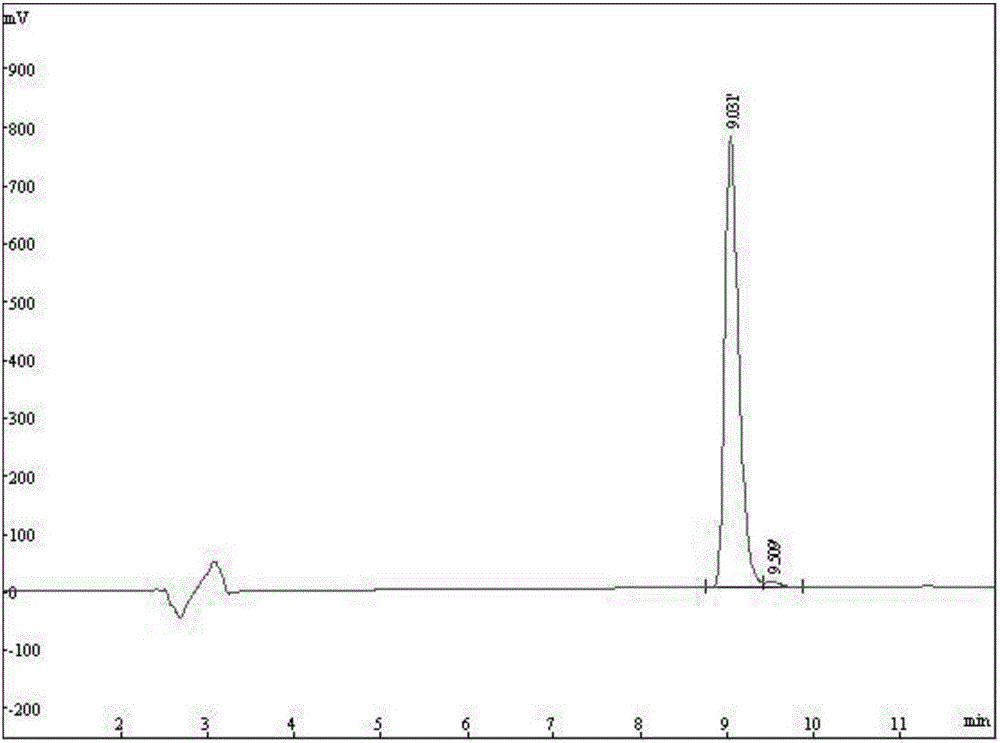
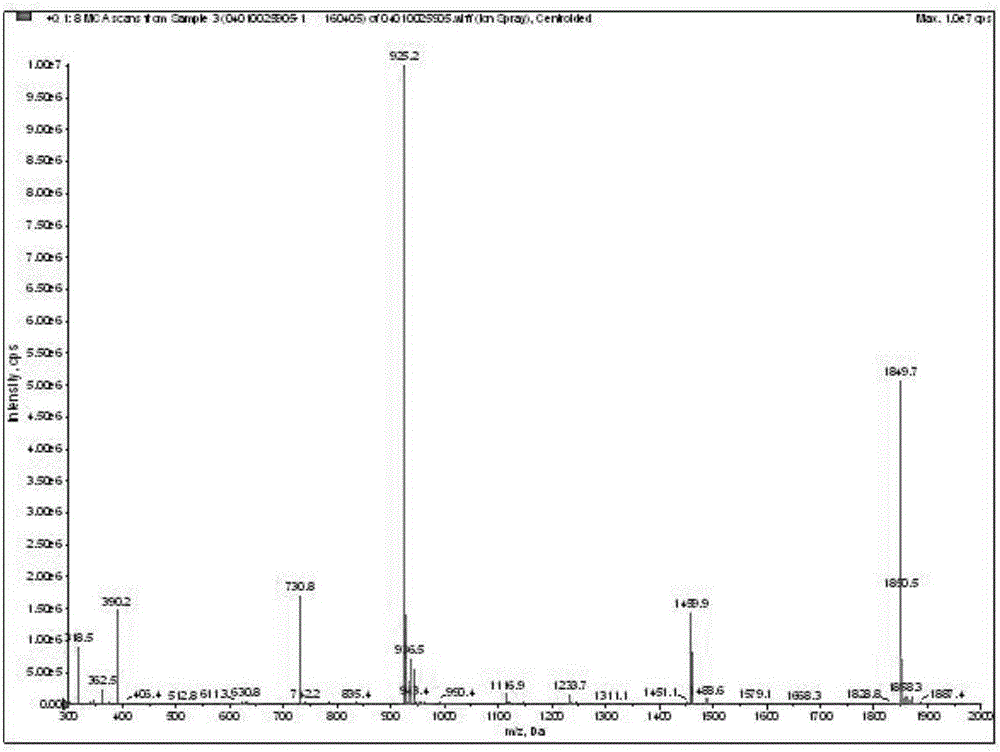
The invention discloses a fluorescent probe for detection of cystatin C and a construction method thereof, relates to the molecular biological and microbiological technical fields, and is characterized in that the construction method comprises the steps: with Cys-C as a target, screening to obtain a Cys-C specific-binding recombinant phage by using a phage surface random displayed 12-mer peptide library, extracting single stranded DNA of the recombinant phage, carrying out sequencing and sequence comparison, to obtain a sequence Gln-Val-Asn-Gly-Leu-Gly-Glu-Arg-Ser-Gln-Gln-Met of a Cys-C specific affinity ligand having the molecular weight of 1346.5, then carrying out solid phase polypeptide synthesis and fluorescence labeling to obtain the fluorescent probe FITC-Acp-Gln-Val-Asn-Gly-Leu-Gly-Glu-Arg-Ser-Gln-Gln-Met having the molecular weight of 1848.7 and specifically bond with the Cys-C. The fluorescent probe and the construction method thereof have the beneficial effects: 1, a brand new 'tool' is provided for specific identification and content detection of the Cys-C; and 2, the fluorescent ligand peptide probe not only can qualitatively identify the Cys-C, but also can quantitatively detect the content of the Cys-C in samples.
Owner:长春技特生物技术有限公司
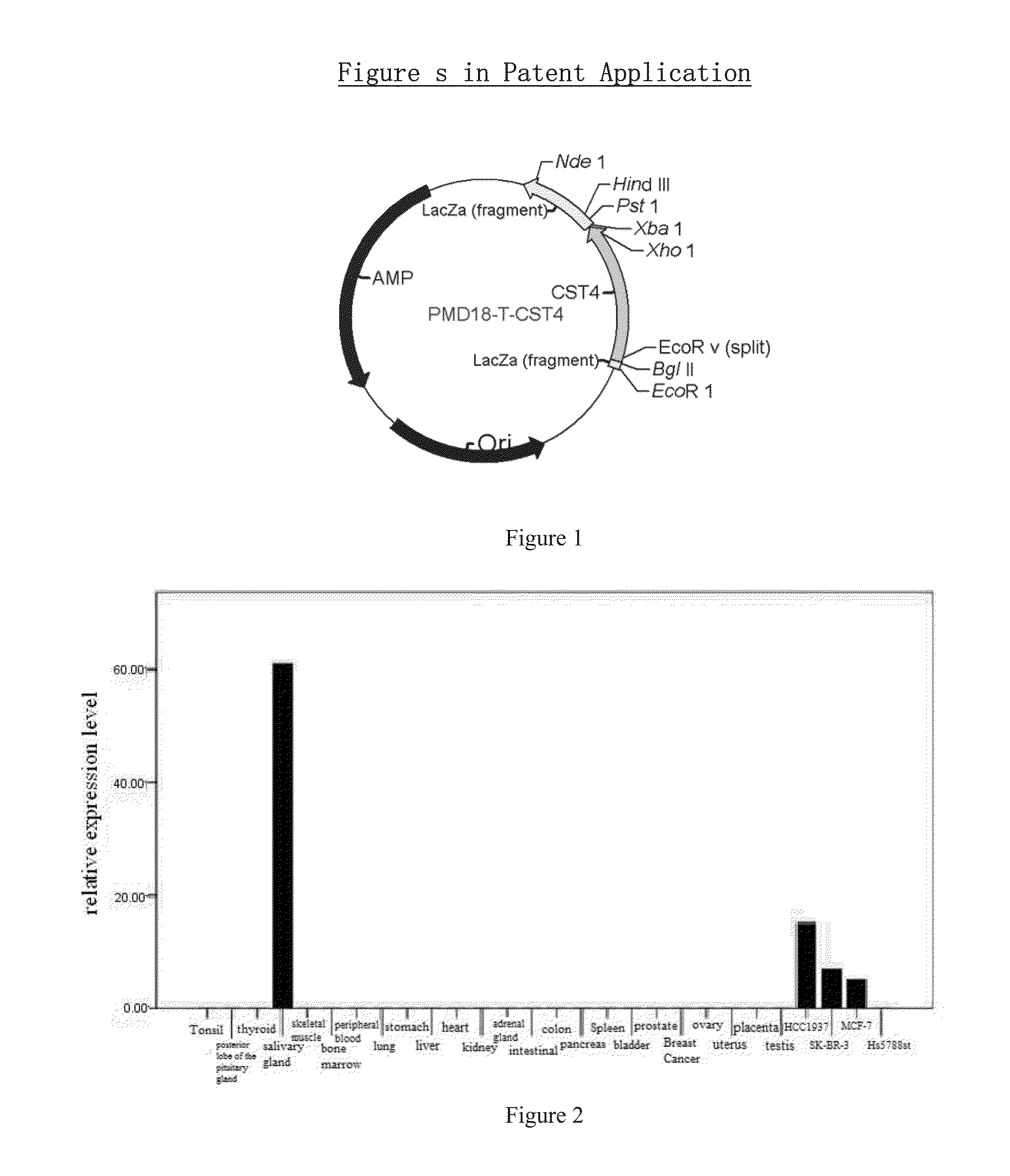
Biomarkers for breast cancer predictions and diagnoses
InactiveUS20150160221A1Improve accuracyEasy to operateMicrobiological testing/measurementImmunoglobulins against animals/humansClinical trialBiologic marker
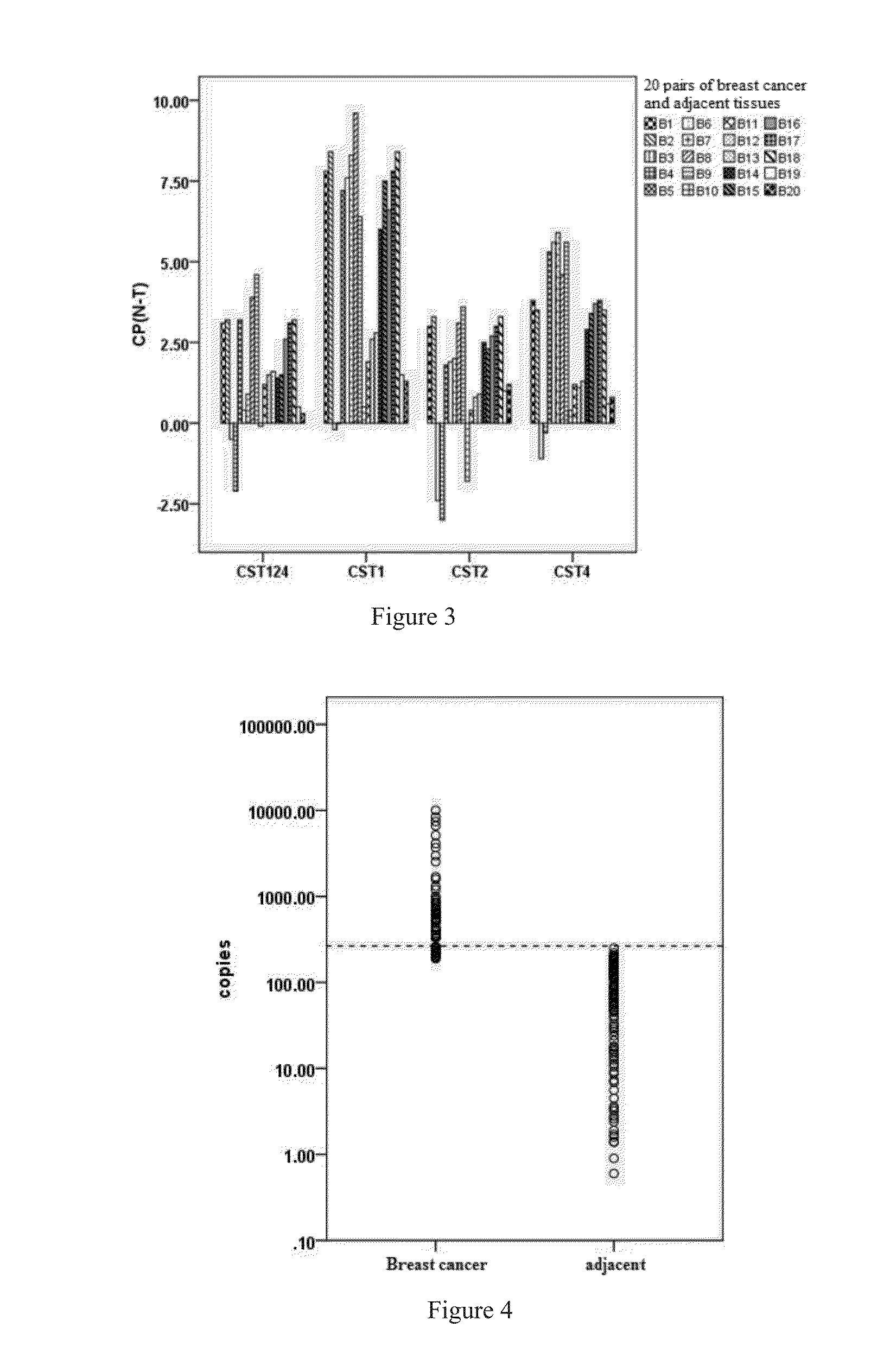
The applications of CST4 gene, mRNA of CST4, cDNA of the splice variants of CST4, the amplicons of the CST4 primers, cystatin S protein coded by CST4 and epitope peptide of cystatin S in the predictions and diagnoses of human breast cancers, is disclosed in this invention. It can be used for the diagnosis and real time monitoring of breast cancers and tumor prognosis predictions. This methods disclosed are verified with large-scale clinical trials, and are of significant reliability and sensitivity.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
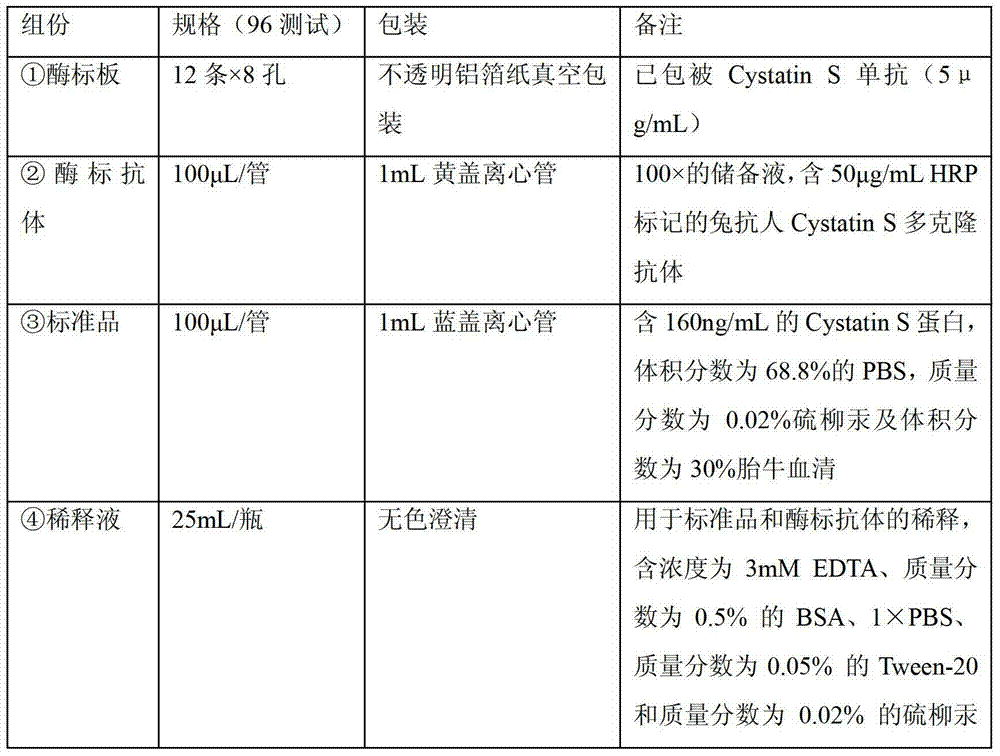
Hybridoma cell capable of secreting anti-cystatin S monoclonal antibody as well as monoclonal antibody and application of hybridoma cell
InactiveCN104558116AStrong specificityHigh sensitivityMicroorganism based processesTissue cultureIntestinal CancerHybridoma cell
The invention discloses a hybridoma cell capable of secreting an anti-Cystatin S monoclonal antibody as well as a monoclonal antibody and an application of the hybridoma cell. The hybridoma cell strains comprise 5D2F2 and 5E4G5 and are preserved in China Center for Type Culture Collection in Wuhan University on June 17, 2014, and the preservation numbers are CCTCC NO:C201416 and CCTCC NO:C201415. The hybridoma cell can secrete the Cystatin S monoclonal antibody, can be specifically combined with Cystatin S recombinant protein, has high potency and good affinity, can be used for specifically detecting Cystatin S and can be used for intestinal cancer diagnosis.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
Methods and compositions for reducing amyloid beta levels
The present invention provides methods for reducing the level of amyloid beta protein in a cell or tissue, the methods generally involving contacting the cell or tissue with an agent that reduces cystatin C levels and / or activity. The present invention provides methods for treating Alzheimer's disease (AD), and methods for treating cerebral angiopathy, in an individual, the methods generally involving administering to an individual having AD a therapeutically effective amount of an agent that reduces cystatin C levels and / or activity. The present invention further provides methods for identifying an agent that reduces cystatin C levels and / or activity.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Recombinant human cystatin C coding gene and expression method
InactiveCN102676533AEasy to operateImprove expression efficiencyFermentationVector-based foreign material introductionEscherichia coliCloning Site
The invention discloses a recombinant human cystatin C coding gene and an expression method. The nucleotide sequence of the coding gene is shown in SEQ ID NO:1, and the expression method of recombinant human cystatin C protein comprises the following steps that the recombinant human cystatin C coding gene is inserted into a polyclone locus of a prokaryotic expression carrier, a recombinant expression plasmid is constructed, the recombinant expression plasmid is converted to an expression host bacterium escherichia coli, and positive clone bacteria are screened out to obtain engineering bacteria; and the engineering bacteria are fermented, induced, separated and purified to obtain the recombinant human cystatin C protein. The recombinant human cystatin C coding gene provided by the invention can express the human cystatin C protein, the operation is convenient, the purity of the human cystatin C protein expressed by the coding gene reaches more than 90%, the expression efficiency is high, and no higher expression efficiency exists currently.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
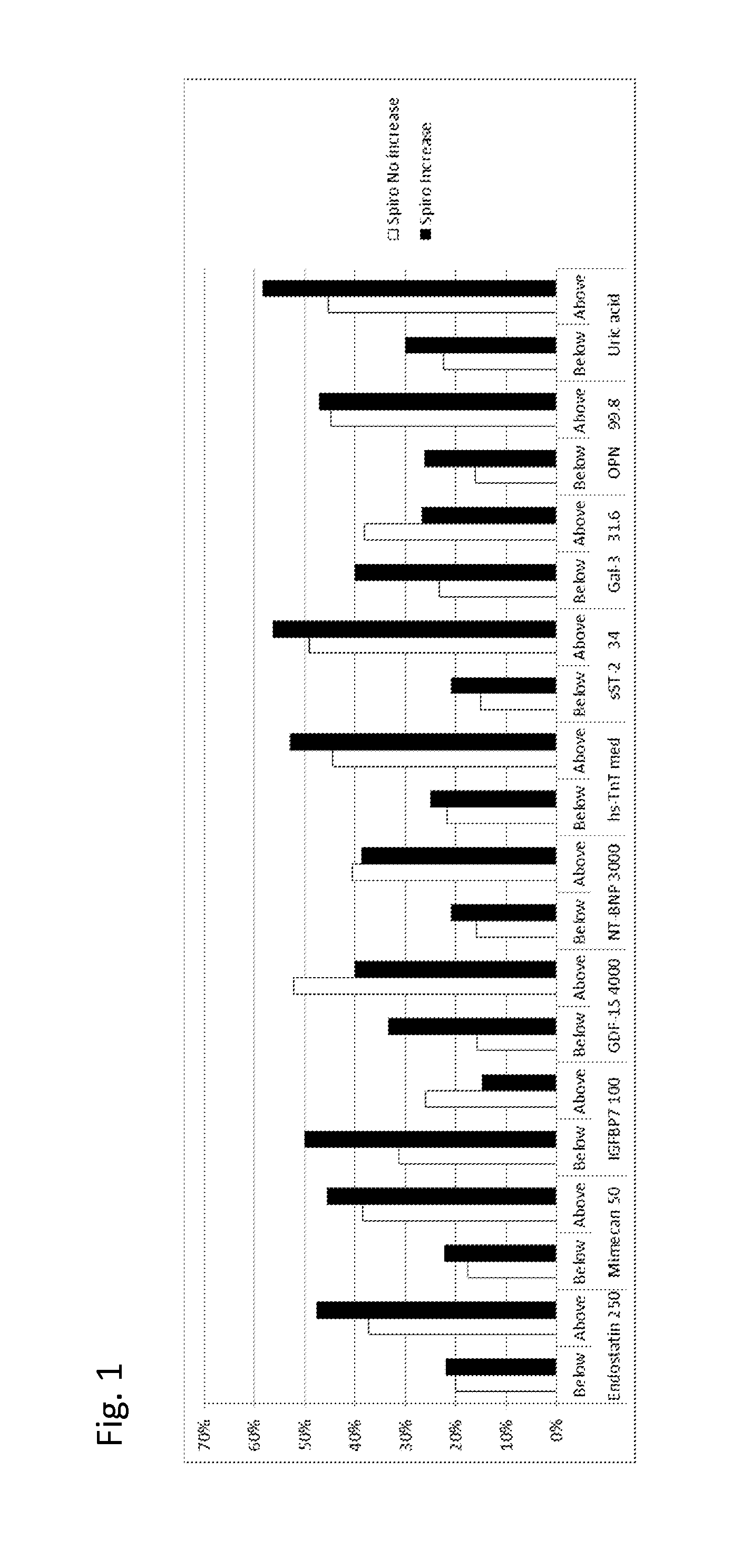
Biomarkers in the selection of therapy of heart failure
InactiveUS20150268251A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBeta blockerBiomarker (petroleum)
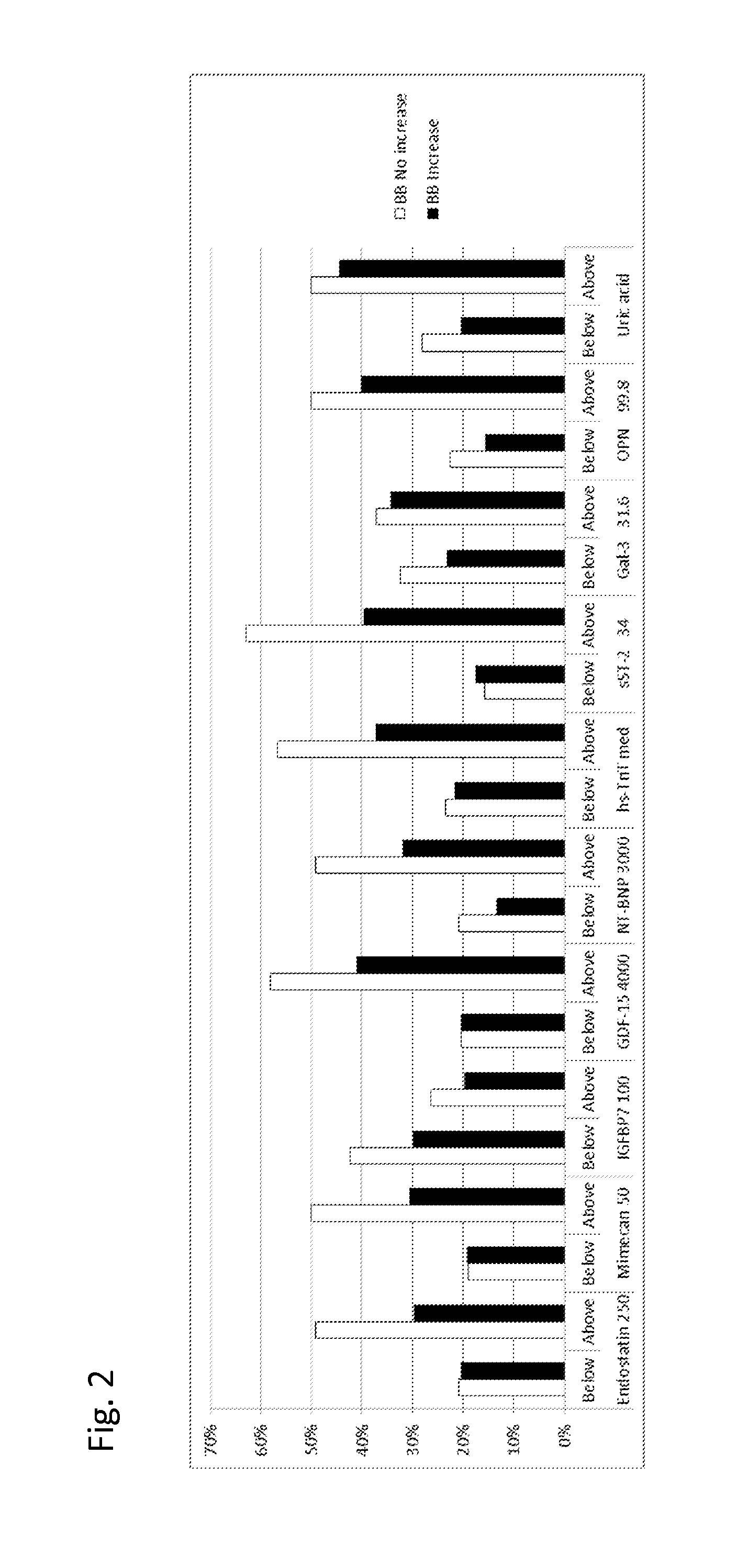
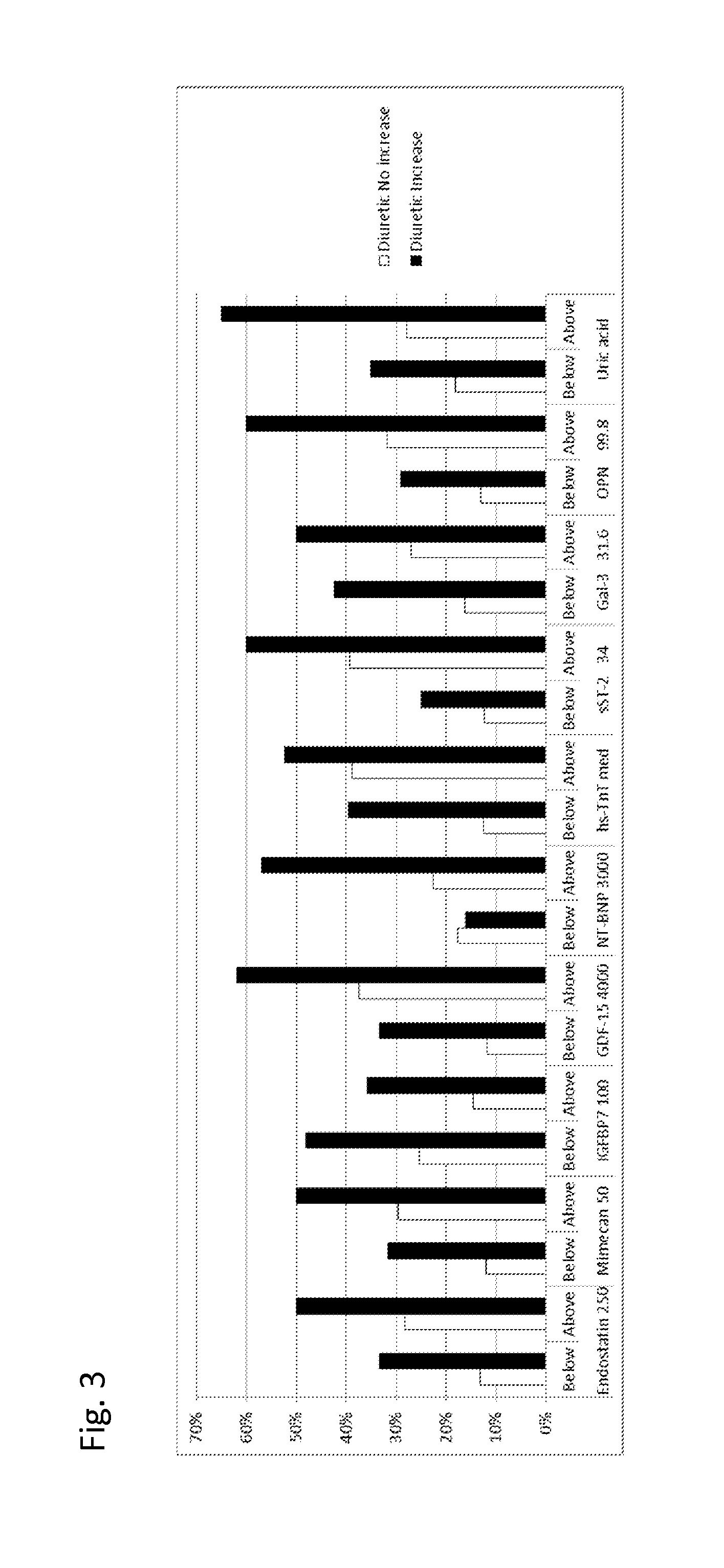
The present invention relates to a method for identifying a subject being eligible to the administration of at least one medicament selected from the group consisting of a beta blocker, an aldosterone antagonist, a diuretic, and an inhibitor of the renin-angiotensin system. The method is based on the determination of the amount of at least one biomarker selected from the group consisting of GDF-15 (Growth Differentiation Factor 15), endostatin, mimecan, IGFBP7 (IGF binding protein 7), a cardiac Troponin, a BNP-type peptide, uric acid, Gal3 (Galectin-3), osteopontin, sST2 (soluble ST2), PlGF, sFlt-1, P1NP, Cystatin C, Prealbumin, and Transferrin in a sample from a subject suffering from heart failure. Further, the method comprises the step of comparing the, thus, determined amount with a reference amount. Further envisaged by the present invention are kits and devices adapted to carry out the method of the present invention. The present invention also relates to a system for identifying a subject being eligible to the administration of at least one medicament as disclosed herein and to reagents and kits used in performing the methods as disclosed herein.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Turbidimetric immunoassay for assessing human cystatin c
ActiveUS20110033950A1Strong and faster signalReduce distractionsDisease diagnosisBiological testingHuman bodyAssay
There is a demand for improved turbidimetric immunoassays for human Cystatin C in biological samples, especially in human clinical samples of body fluids. The present invention provides a turbidimetric immunoassay method and reagent set enabling measurement of human Cystatin C by turbidimetric methods, resulting in a surprisingly stronger and faster turbidimetric signal than in the present state of the art. The increased and faster signal is accomplished by the use of new reagents and compositions, and enables shorter assay times and kinetic reading with a stronger signal, improving overall assay speed and quality. Improved robustness to lipid interference and improved linearity is achieved.
Owner:GENTIAN AS
Cystatin C detection reagent and method
ActiveCN107228940AEasy to operateImprove stabilityColor/spectral properties measurementsSucroseMicrosphere
The invention relates to the technical field of in-vitro diagnostic reagents and particularly relates to a cystatin C detection reagent and method. The detection reagent is composed of a reagent 1 and a reagent 2. The reagent 1 comprises ammonium chloride, polyoxyethylene lauryl ether, sodium chloride, PEG 6000, a preservative and water. The reagent 2 comprises latex microspheres coated with a cystatin C monoclonal antibody, a Tris-HCl buffer solution, sucrose, gelatin, NP30, a preservative and water. The detection reagent has high detection result accuracy, a good precision, a wide linear range, good specificity, good stability and strong anti-interference ability, can be conveniently detected on a full-automatic biochemical analyzer, has a wide application range and is easy to promote.
Owner:吉林省汇酉生物技术股份有限公司
Construction, expression and purification of human cystatin C eucaryon expression vector
The invention discloses a method for expressing and purifying recombinant human cystatin C proteins by using a eukaryon expression system. Because posttranslational modification and other mechanisms are lacked in a pronucleus expression system, the expressed and purified proteins have the defects of low activity and large structural difference as compared with the natural proteins, which results in that a cystatin C detection agent is difficult in the establishment process by using a monoclonal antibody prepared from Escherichia coli source recombinant cystatin C proteins. In the method, the defects of the pronucleus expression are overcome by utilizing the eukaryon expression system so as to obtain the high-activity recombinant human cystatin C protein, and a powerful basis is provided for obtaining a high-quality antibody.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
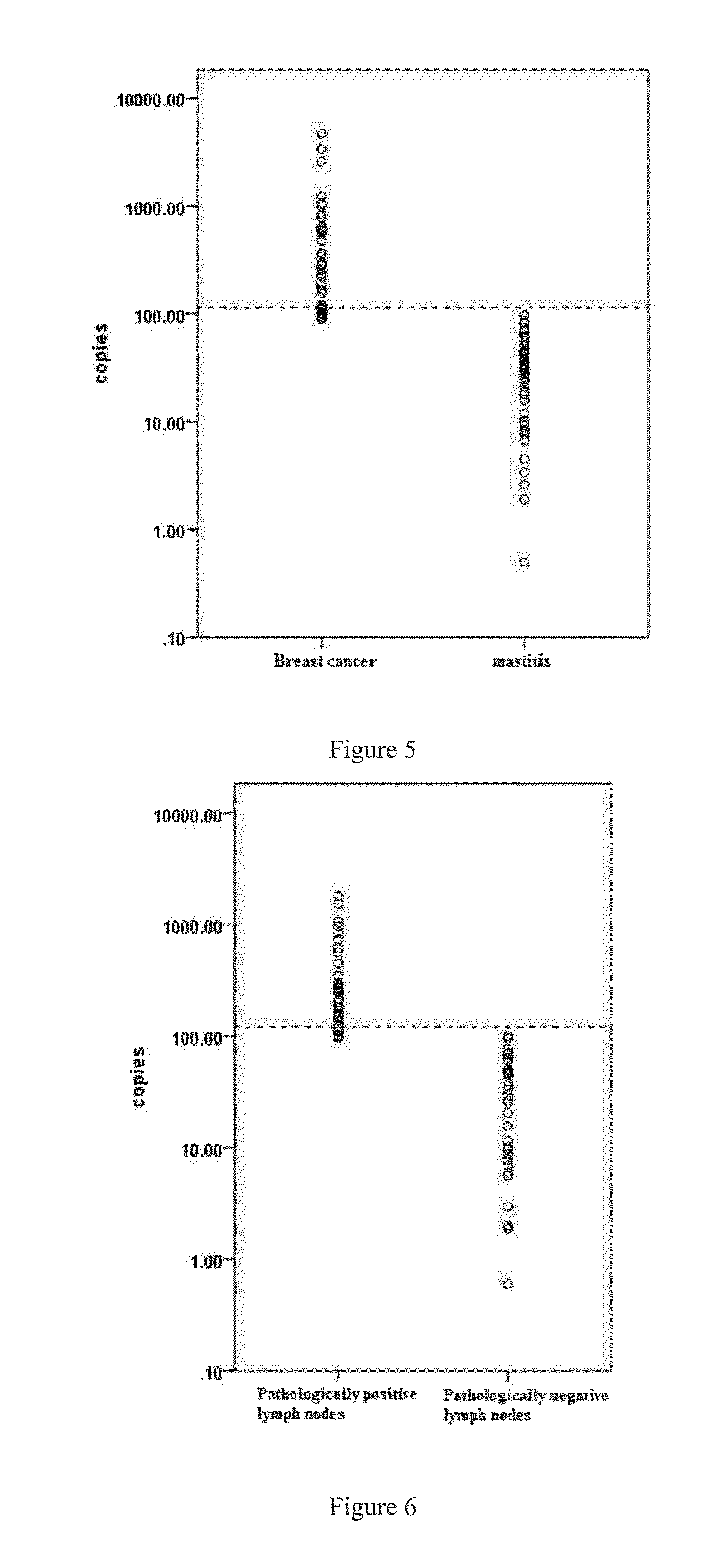
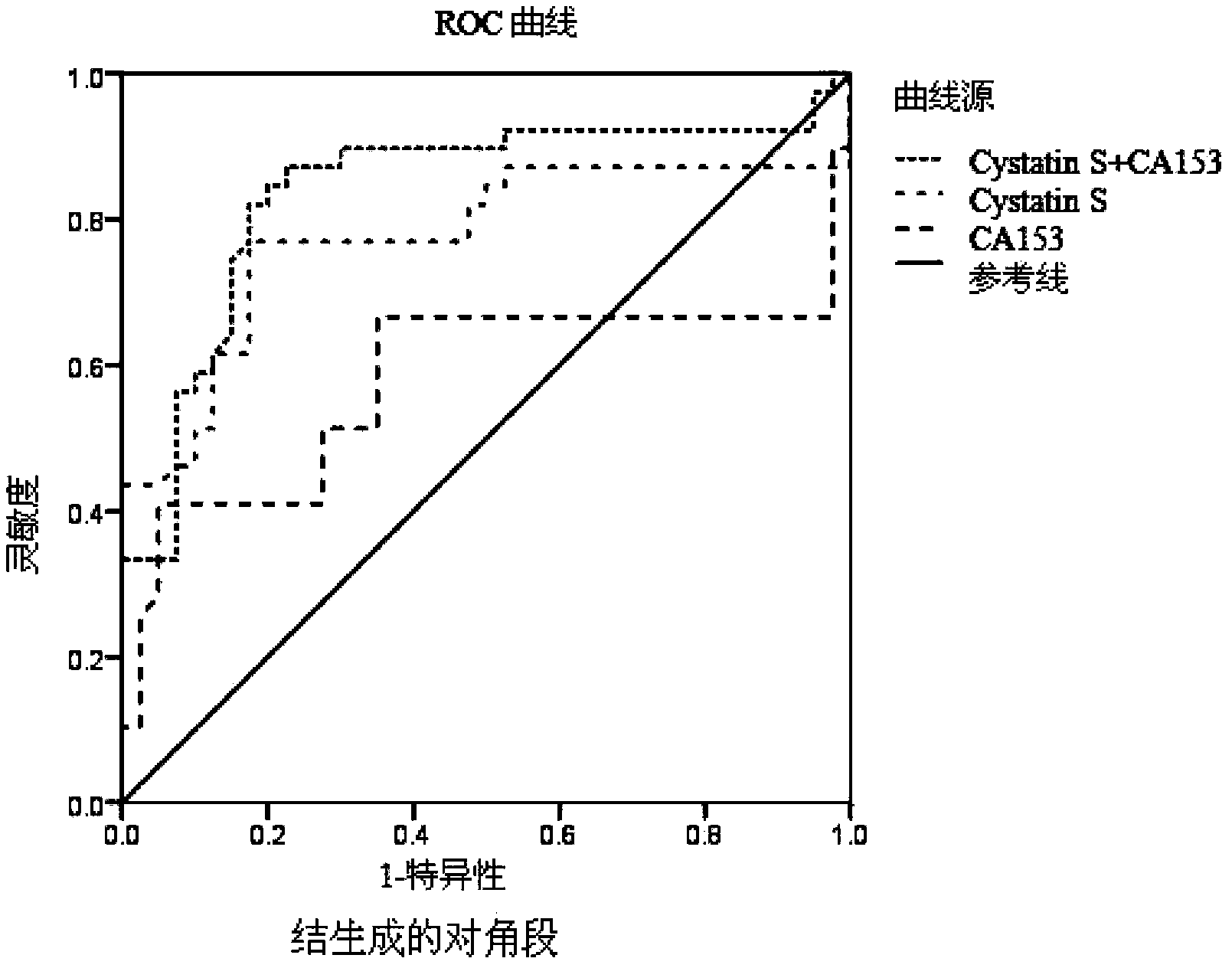
Application of Cystatin S and CA15-3 in preparation of markers for diagnosing and predicting breast cancer
The invention discloses combined application of cysteine proteinase inhibitor S (Cystatin S) and carbohydrate antigen 15-3 (CA15-3) and in particular discloses application of Cystatin S and CA15-3 in preparation of markers for diagnosing and predicting breast cancer. The invention also discloses a trapping agent of breast cancer markers and a kit comprising the trapping agent. The kit has the advantages of good specificity and high sensitivity and can be applied to early diagnosis of the breast cancer, therapeutic effect evaluation in a breast cancer treating process and monitoring of transfer and recurrence after treating the breast cancer; diagnosis results can be earlier than clinical symptoms.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
Hybridoma cells capable of secreting mouse anti cystatin C monoclonal antibodies, secreted monoclonal antibodies and use of monoclonal antibodies
ActiveCN107828739AHigh sensitivityStrong specificityMicroorganism based processesDisease diagnosisSerum igeSerum free
The invention relates to two strains of hybridoma cells capable of secreting mouse anti CysC monoclonal antibodies; the two strains of hybridoma cells of the mouse anti CysC monoclonal antibodies arecultured by an in vitro serum-free culture method, and the monoclonal antibodies are prepared. The invention also relates to a use of the monoclonal antibodies for kits for detection of CysC in humanserum, plasma or whole blood. The monoclonal antibodies have the advantages of high sensitivity, strong specificity, good stability and high purity.
Owner:SICHUAN ANKERUI NEW MATERIAL CO LTD
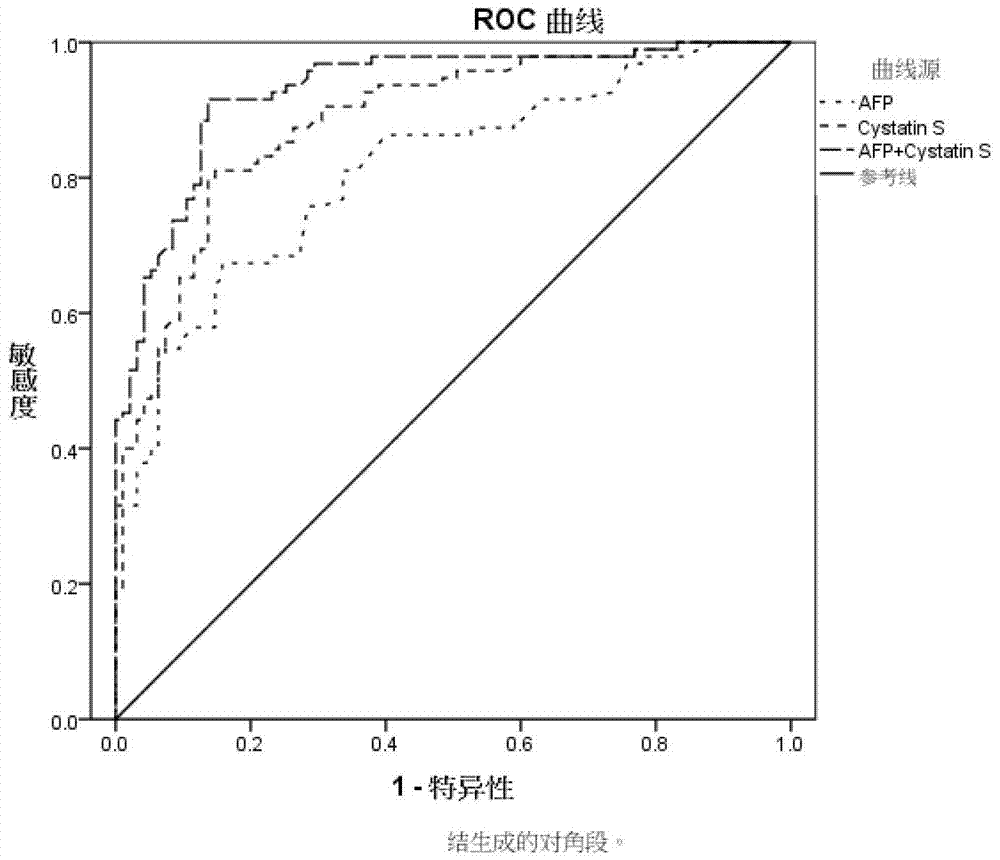
Application of Cystatin S and AFP to prepare marker for diagnosing and indicating liver cancer
The invention discloses combined application of Cystatin S and alpha fetoprotein (AFP), and specifically discloses application of Cystatin S and AFP to prepare a marker for diagnosing and indicating liver cancer. The invention also discloses a trapping agent of the liver-cancer marker, and a liver-cancer detection kit is formed by combining the trapping agent and routine reagents. The kit has the advantages of good specificity, high sensitivity and the like, is applicable to early diagnosis on liver cancer, assessment on treatment effect during treatment and monitoring on metastasis and recurrence after treatment is finished, and the diagnosis result is earlier than clinic symptoms.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
Recombinant cystatin C protein and application of recombinant cystatin C protein to detection kit
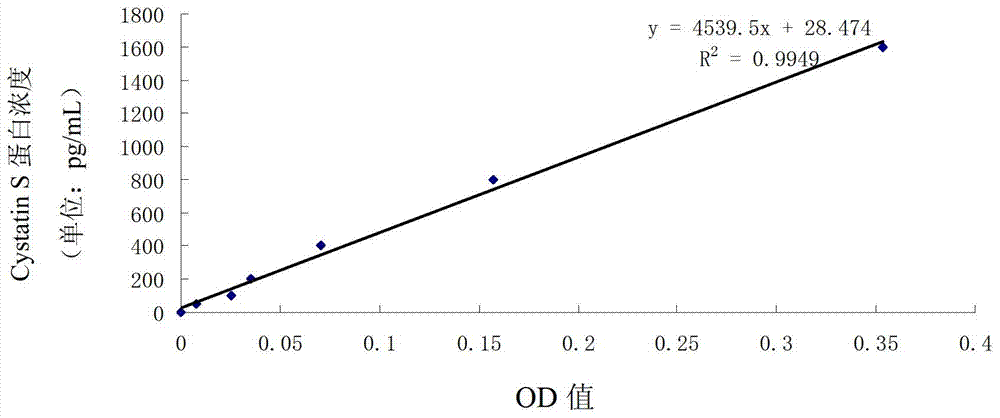
PendingCN109975552AAccurate detection of CysC contentHigh expressionDisease diagnosisBiological testingEscherichia coliHeterologous
The invention relates to a recombinant cystatin C protein (rhCysC) and the application of the recombinant cystatin C protein to a detection kit for detecting serum cystatin C (CysC) and belongs to thefield of in vitro diagnosis of medical immunology. According to the recombinant cystatin C protein (rhCysC) and the application of the recombinant cystatin C protein of the invention, the gene sequence of coded rhCysC, which is obtained through specially carrying out codon optimization on an escherichia coli expression system, is provided, wherein the sequence is as shown as SEQ ID NO: 2. With the sequence transferred, the expression efficiency of heterologous genes in a host bacterium can be remarkably improved. The recombinant expression method of the rhCysC has the characteristics of shortperiod, large expression quantity and low cost; the rhCysC prepared by the method can be used as an effective immunogen for the preparation of rabbit polyclonal antibodies. With the polyclonal antibodies applied to the preparation of a latex-enhanced immunoturbidimetric detection kit, the content of CysC in serum can be accurately detected. Compared with a commercially available product, the recombinant cystatin C protein (rhCysC) has better repeatability and higher accuracy, and shows a good clinical application prospect.
Owner:ZONHON BIOPHARMA INST
Synthetic avian-free egg white substitute and method of making same
In some embodiments, the present disclosure relates to an avian-free egg white composition including 45-63% Ovalbumin, 9-15% Ovotransferrin, 0-15% Ovomucoid, 3-5% Ovoglobulin G2, 3-5% Ovoglobulin G3, 2.5-5% Ovomucin, 3-5% Lysozyme, 1-2% Ovoinhibitor, 0.8-1.5% Ovoglycoprotein, 0.6-1.0% Flavoprotein, 0.3-0.8% Ovomacroglobulin, 0.02-0.1% Avidin, and 0.02-0.1% Cystatin. In some embodiments, the composition includes an edible yeast and one or more of the preceding proteins. In some embodiments, the avian-free egg white further includes one or more of: flavor enhancers, calcium supplements, added vitamins, and a gelling agent. In some embodiments, the present disclosure pertains to a non-allergenic egg-white composition for human consumption. In some embodiments, the present disclosure pertains to methods of making the avian-free egg-white composition.
Owner:CHALLAKERE KEDARNATH KRISHNAMURTHY
Application of cystatin S
ActiveCN103911426AHigh sensitivityStrong specificityMicrobiological testing/measurementBiological material analysisPancreas CancersAfter treatment
The invention discloses new application of cystatin S, and specifically discloses application of cystatin S to prepare a marker for diagnosing and indicating liver cancer, gastrointestinal stromal tumor, pancreas cancer, esophagus cancer or stomach cancer. The invention also discloses a trapping agent of the marker for diagnosing and indicating liver cancer, gastrointestinal stromal tumor, pancreas cancer, esophagus cancer or stomach cancer, and the trapping agent is applied to prepare a kit for diagnosing and indicating liver cancer, gastrointestinal stromal tumor, pancreas cancer, esophagus cancer or stomach cancer. The prepared kit has the advantages of being good in specificity, high in sensitivity and the like, is applicable to early diagnosis on liver cancer, gastrointestinal stromal tumor, pancreas cancer, esophagus cancer or stomach cancer, assessment on treatment effect during treatment and monitoring on metastasis and recurrence after treatment is finished.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
A kind of cystatin c detection kit and preparation method thereof
ActiveCN103645323BHigh sensitivityGood precisionBiological material analysisBiological testingMedicinePolystyrene
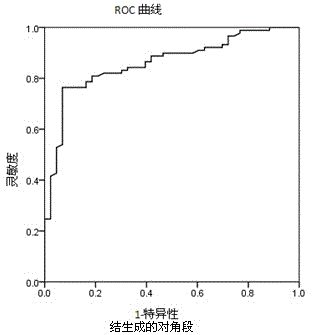
The invention relates to the field of medical immunology in vitro diagnosis, and especially provides a detection kit for detecting cystatin C in serum. The detection kit comprises a reagent R1, a reagent R2 and calibration materials. The ingredients of the reagent R1 are a Tris buffer with a concentration of 0.01-0.05mol / L, PEG 6000 with a concentration of 10-40g / L, NaN3 with a concentration of 0.5-1.5g / L, and sodium chloride with a concentration of12-20g / L, and the pH value is 8.0-8.5. The ingredients of the reagent R2 are a Tris buffer with a concentration of 0.01-0.02mol / L, and polystyrene latex granules coated by goat-anti-human cystatin C polyclonal antibodies with a concentration of 0.5-3.0 g / L. The calibration materials are six gradient standards with cystatin C and the contents of cystatin C are 0.1, 0.5, 1.0, 2.0, 4.0 and 8.0mg / L. The solution is a Tris buffer with a concentration of 0.01mol / L. The kit has simple composition, good stability and high accuracy.
Owner:浙江夸克生物科技有限公司
Synthetic avian-free egg white substitute and uses thereof
In some embodiments, the present disclosure relates to an avian-free egg white composition comprising 45-63% Ovalbumin, 9-15% Ovotransferrin, 0-15% Ovomucoid, 3-5% Ovoglobulin G2, 3-5% Ovoglobulin G3, 2.5-5% Ovomucin, 3-5% Lysozyme, 1-2% Ovoinhibitor, 0.8-1.5% Ovoglycoprotein, 0.6-1.0% Flavoprotein, 0.3-0.8% Ovomacroglobulin, 0.02-0.1% Avidin, and 0.02-0.1% Cystatin. In some embodiments, the composition comprises an edible yeast and one or more of the preceding proteins. In some embodiments, the avian-free egg white further comprises one or more of: flavor enhancers, calcium supplements, added vitamins, and a gelling agent. In some embodiments, the present disclosure pertains to a non-allergenic egg-white composition for human consumption. In some embodiments, the present disclosure pertains to methods of making the avian-free egg-white composition.
Owner:CHALLAKERE KEDARNATH KRISHNAMURTHY
Application of Cystatin S and CYFRA21-1 (Cytokeratin Fragement 21-1) in preparation of markers for diagnosing and predicting esophagus cancer
The invention discloses combined application of cysteine proteinase inhibitor S (Cystatin S) and cytokeratin-19-fragment 21-1 (CYFRA21-1) and in particular discloses application of Cystatin S and CYFRA21-1 in preparation of markers for diagnosing and predicting esophagus cancer. The invention also discloses a trapping agent of esophagus cancer markers and a kit comprising the trapping agent. The kit has the advantages of good specificity and high sensitivity and can be applied to early diagnosis of the esophagus cancer, therapeutic effect evaluation in a breast cancer treating process and monitoring of transfer and recurrence after treating the breast cancer; diagnosis results can be earlier than clinical symptoms.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
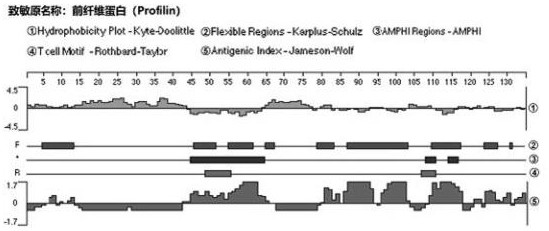
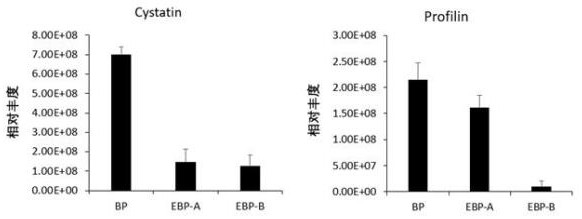
Method for evaluating sensitization of natural bee pollen and enzymic wall-broken bee pollen
ActiveCN113341036AAllergenicity VerificationImprove accuracyComponent separationBiotechnologyProfilin
The invention relates to the technical field of food, in particular to a method for evaluating sensitization of natural bee pollen and wall-broken bee pollen based on Cystatin and Profibrin indexes. According to the invention, Cystatin and Profilin are used as sensitization evaluation indexes of natural bee pollen or wall-broken bee pollen for the first time, and a specific evaluation method is provided according to the evaluation indexes. Meanwhile, the invention further optimizes the application of the dot immunoblotting method in determining the sensitization of the natural bee pollen and the wall-broken bee pollen, and the dot immunoblotting method is used as a mutual verification means. According to the evaluation method, the sensitization of the natural bee pollen and the wall-broken bee pollen can be rapidly and accurately evaluated, and an evaluation basis is provided for standardized production and quality control of the bee pollen.
Owner:BEE RES INST CHINESE ACAD OF AGRI SCI
Combined application of cystatin S and carcinoembryonic antigen
ActiveCN103913574AEasy to useGood repeatabilityImmunoglobulins against animals/humansDisease diagnosisStromal tumorLymphatic Spread
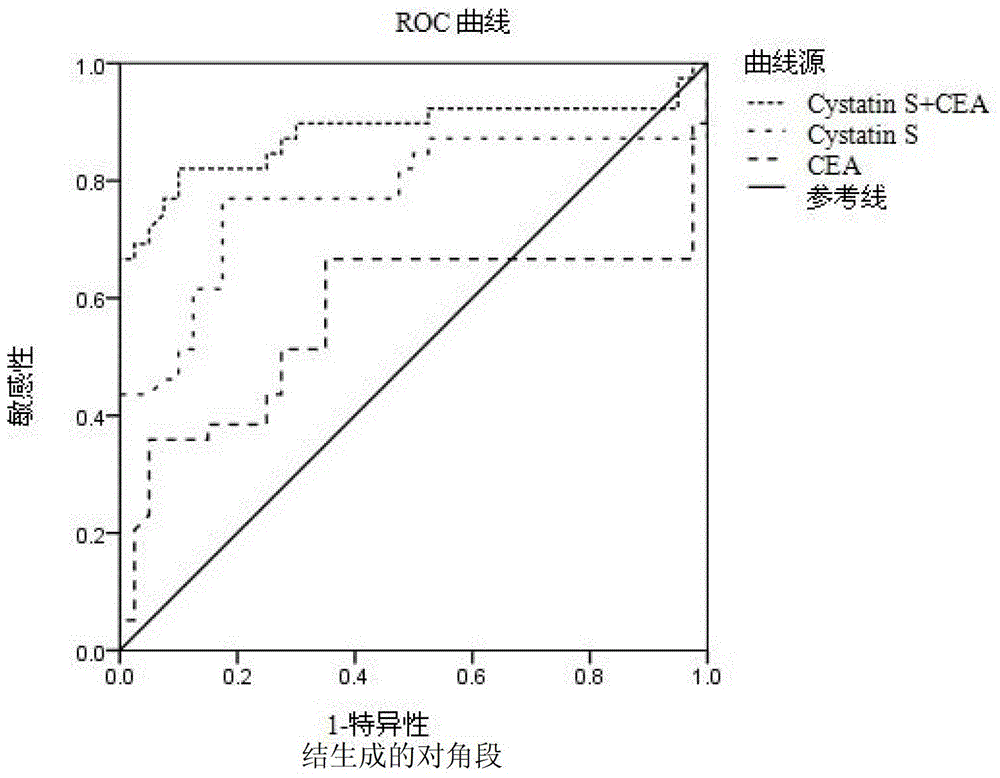
The invention discloses combined application of cystatin S and carcinoembryonic antigen (CEA), and specifically discloses application of cystatin S and CEA to prepare a marker for diagnosing and indicating stomach cancer or gastrointestinal stromal tumor. The invention also discloses a trapping agent of the marker of stomach cancer or gastrointestinal stromal tumor and a kit containing the trapping agent. The kit has the advantages of being good in specificity, high in sensitivity and the like, is applicable to early diagnosis on stomach cancer or gastrointestinal stromal tumor, assessment on treatment effect during treatment and monitoring on metastasis and recurrence after treatment is finished, and the diagnosis result is earlier than clinic symptoms.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
SILAC-based mass spectrum method for absolute quantification of serum cystatin C
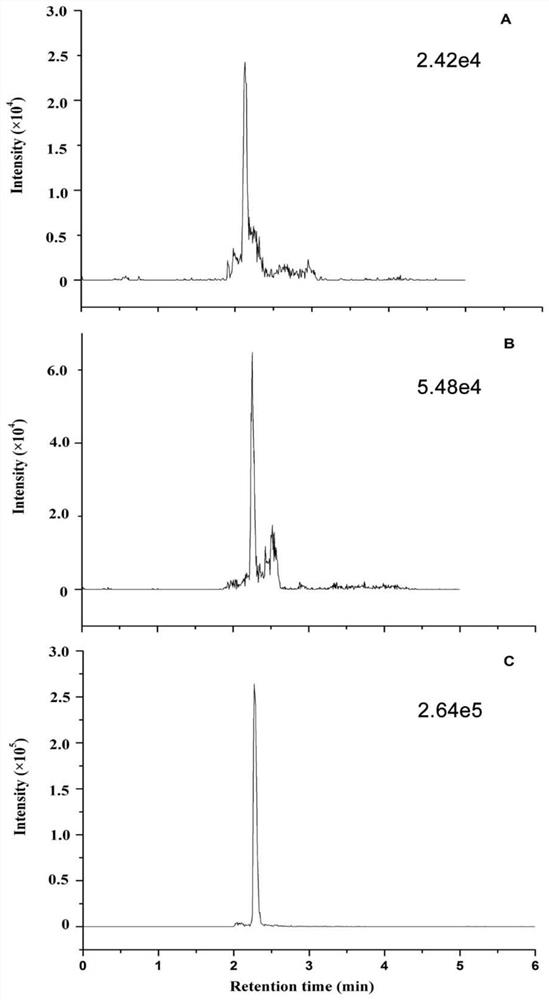
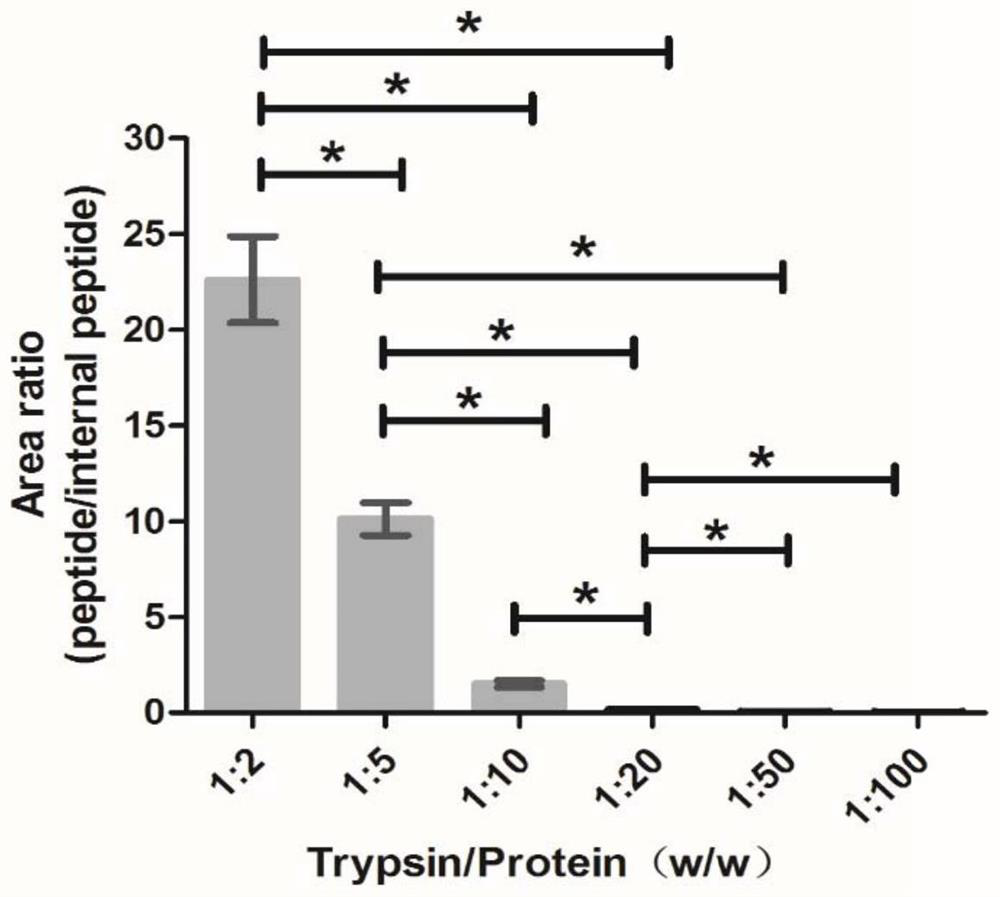
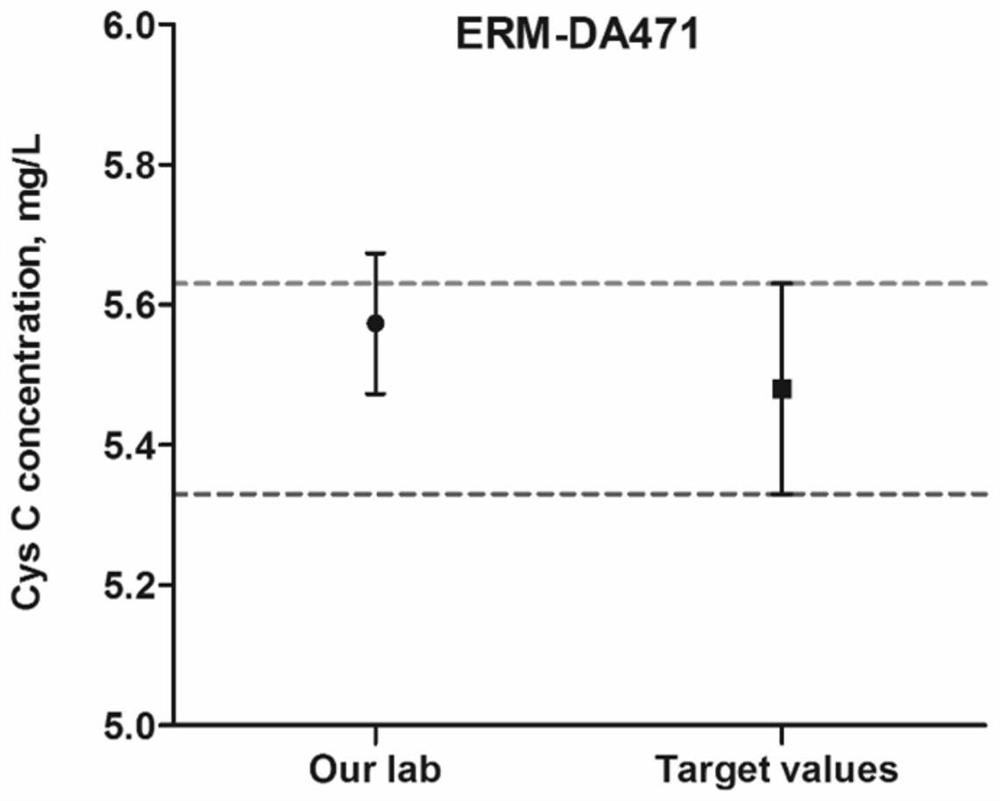
PendingCN114705849AThe result error is smallImprove extraction efficiencyComponent separationBiological material analysisEpoxyEnzyme digestion
The invention relates to the technical field of mass spectrum detection, in particular to a mass spectrum method for absolute quantification of serum cystatin C based on SILAC. The invention discloses establishment and performance evaluation of an absolute quantification method for human serum cystatin C based on SILAC, the absolute quantification of a target protein is carried out by using a complete CysC protein marked by 15N as an internal standard for the first time, and result errors caused by sample loss, incomplete enzyme digestion and the like in a sample pretreatment process are greatly reduced. Specific anti-M270 epoxy resin magnetic bead co-immunoprecipitation is adopted in sample pretreatment, the extraction efficiency is higher than that of an existing method, and the actual sample detection sensitivity and the anti-interference capacity are improved. According to the invention, a CysC national standard substance GBW09870 is combined with an amino acid analysis method to carry out quantity value traceability of CysC detection in serum for the first time.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Quantitative immune colloidal gold detection card and kit for urocystatin C, urine microalbumin and urine creatinine
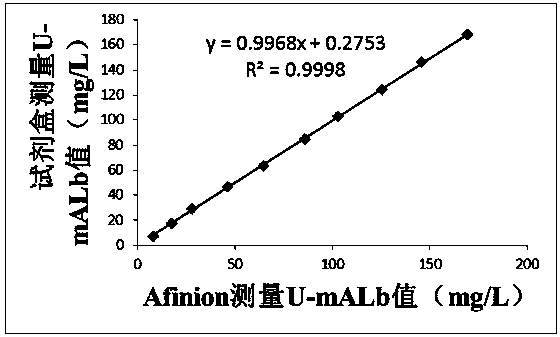
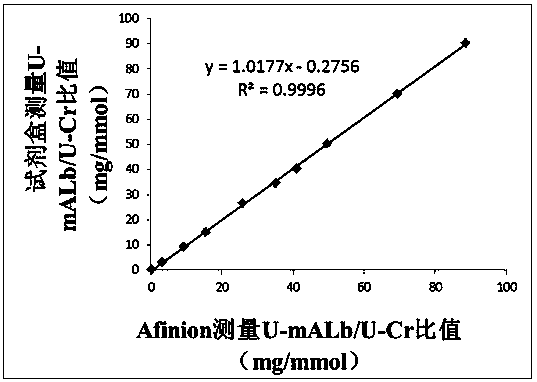
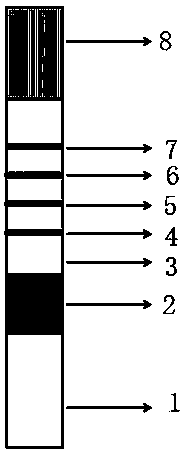
PendingCN110988365AImprove the detection rateImprove detection accuracyDisease diagnosisBiological testingElevated creatinineColloidal au
The invention discloses a quantitative immune colloidal gold detection card for urine cystatin C, urine microalbumin and urine creatinine. The detection card comprises a buckle box, a bottom plate, and a sample pad, a combination pad, a chromatographic membrane and a sample absorption pad which are sequentially adhered to the bottom plate. The combination pad is coated with a colloidal gold labeled cystatin C antibody I, a urine microalbumin antibody I, a urine creatinine antibody I and a chicken IgY. A first detection line coated with a cystatin C antibody II, a second detection line coated with a urine microalbumin antibody II, a third detection line coated with a urine creatinine antigen modified by BSA, and a quality control line coated with goat anti-chicken IgY are arranged on the chromatographic membrane. Compared with single detection of U-mALb and U-Cr values, the U-mAlb / U-Cr ratio obtained in the invention can more sensitively reflect the degree of renal function impairment,and the accuracy of the detection result is improved.
Owner:HUNAN UNIV OF TECH
Diagnosis and monitoring of renal failure using peptide biomarkers
InactiveUS8673574B2Accurate descriptionSugar derivativesPeptide/protein ingredientsChronic kidney failureKidney injury
Methods for the determination of renal failure, especially chronic renal failure and acute kidney injury, by measurement of peptide or protein biomarkers are described. The methods are useful to determine stages of renal failure, especially the early stages such as stage 1, 2, and 3 of chronic renal failure and stages R and I of acute kidney injury. Furthermore there are described peptides and test kits used in the invention. The described methods are intended to replace or complement the measurement of creatinine and / or cystatin C and / or NGAL for diagnosis of renal failure.
Owner:PXBIOSCI
Application of snow tea extract to preparation of medicine for preventing and treating zymloid protein diseases
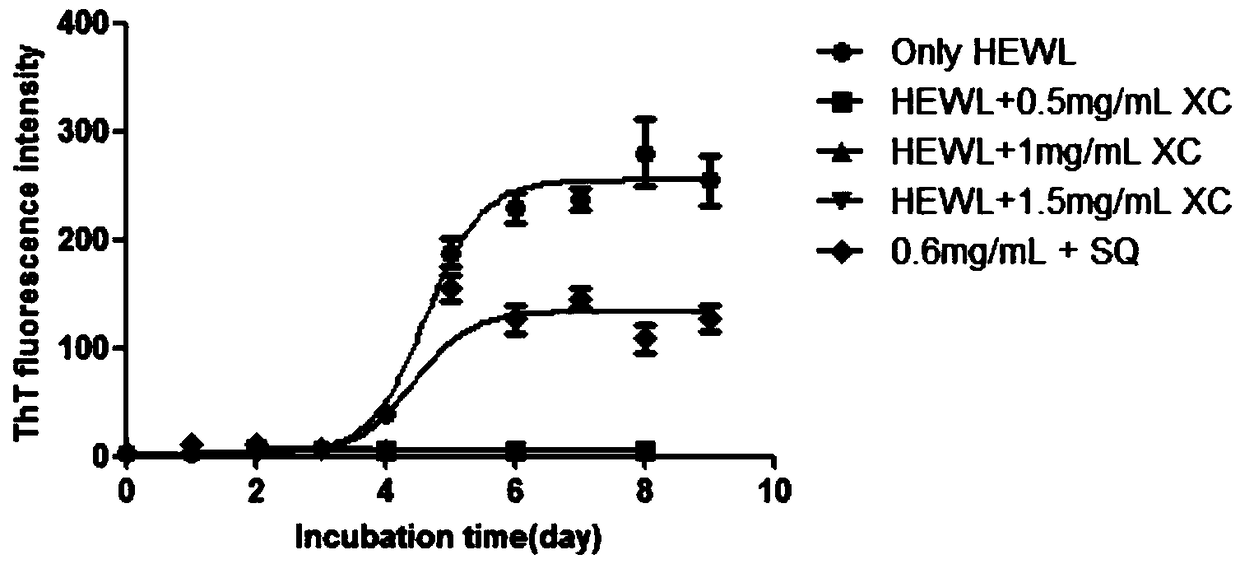
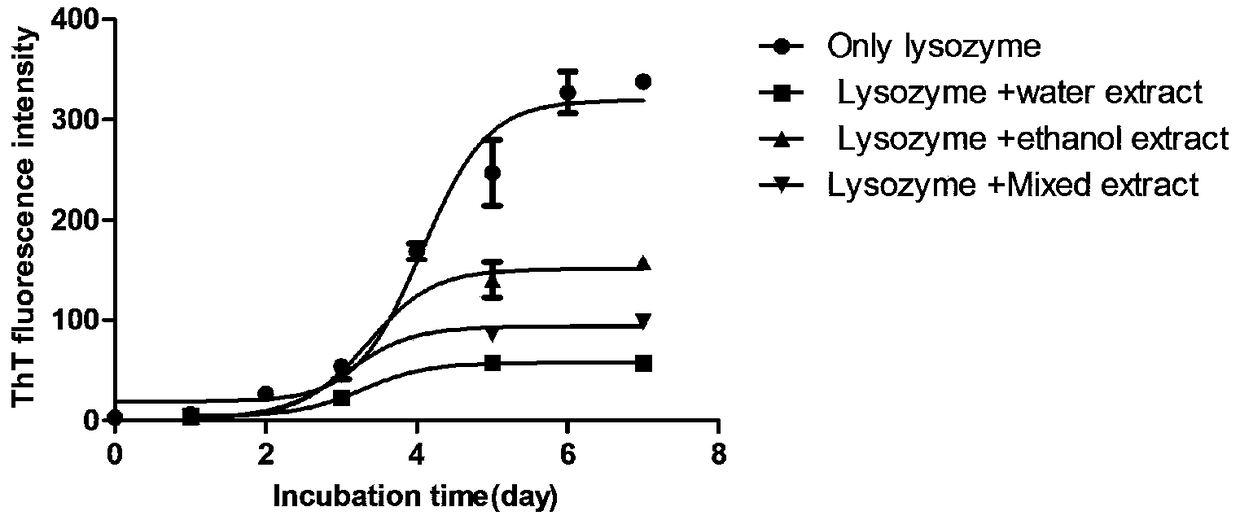
InactiveCN108785334AReduce the amount requiredProlonged nucleation periodNervous disorderLichen medical ingredientsPichia pastorisAmyloid beta
The invention relates to application of a snow tea extract to preparation of a medicine for preventing and treating zymloid protein diseases. The influence on zymloid protein fiber formation by the snow tea extract is mainly explored, a new effective treatment medicine for preventing and treating zymloid deposition diseases is expected to find, and support is provided for medicinal research on thesnow tea. Typical zymloid protein chicken lysozyme is mainly subjected to in-vitro culture to form fiber and secreted protein of a recombinant pichia pastoris strain of chicken cystatin forms fiber.The result indicates that the snow tea extract achieves the effect of inhibiting the zymloid protein from forming the fiber; furthermore, in the water extract group, the effect is more significant andthe IC50 value is about 0.018 mg / mL. The bacterial level protein expression experiment indicates that the snow tea extract can inhibit a chicken cystatin zymloid mutant I66Q from forming aggregate extracellularly in a concentration dependency way; and when the extract concentration reaches to 1.5 mg / mL, a certain inhibition effect can be generated on oligomerization and aggregation of the secreted protein.
Owner:LIAONING UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com