Construction, expression and purification of human cystatin C eucaryon expression vector
A technology of eukaryotic expression vector and expression vector, which is applied in the field of expression and purification of recombinant human cystatin C, which can solve the problems of poor antibody specificity, low sensitivity, and poor specificity of natural cystatin C
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Construction, expression and purification of cystatin C eukaryotic expression vector
[0029] (1) Construction of cystatin C eukaryotic expression vector.
[0030] Primers were designed according to the sequence of cystatin C:
[0031] upstream primer ATCG GGATCC TCCAGTCCTGGCAAG
[0032] downstream primer C GGAATTC TTA ATGATGATGATGATGATG GGC GTCCTGACAGGT
[0033] Among them, a BamHI restriction site was introduced into the upstream primer, and an EcoRI restriction site and a 6×histidine tag (indicated by the underlined part) were introduced into the downstream primer. Carry out PCR amplification with 56 degrees as the annealing temperature. After the gel is recovered, it is digested, connected with the pcDNA3.1(+) vector digested with BamHI and EcoRI and transformed into Escherichia coli DH5a. After PCR identification, select positive recombinants to carry out Sequencing identification.
[0034] (2) Cystatin C expression in CHO cells
[0035] The correct clon...
Embodiment 2
[0037] Use Beijing Jiuqiang Cystatin C Detection Kit to measure the purified protein:
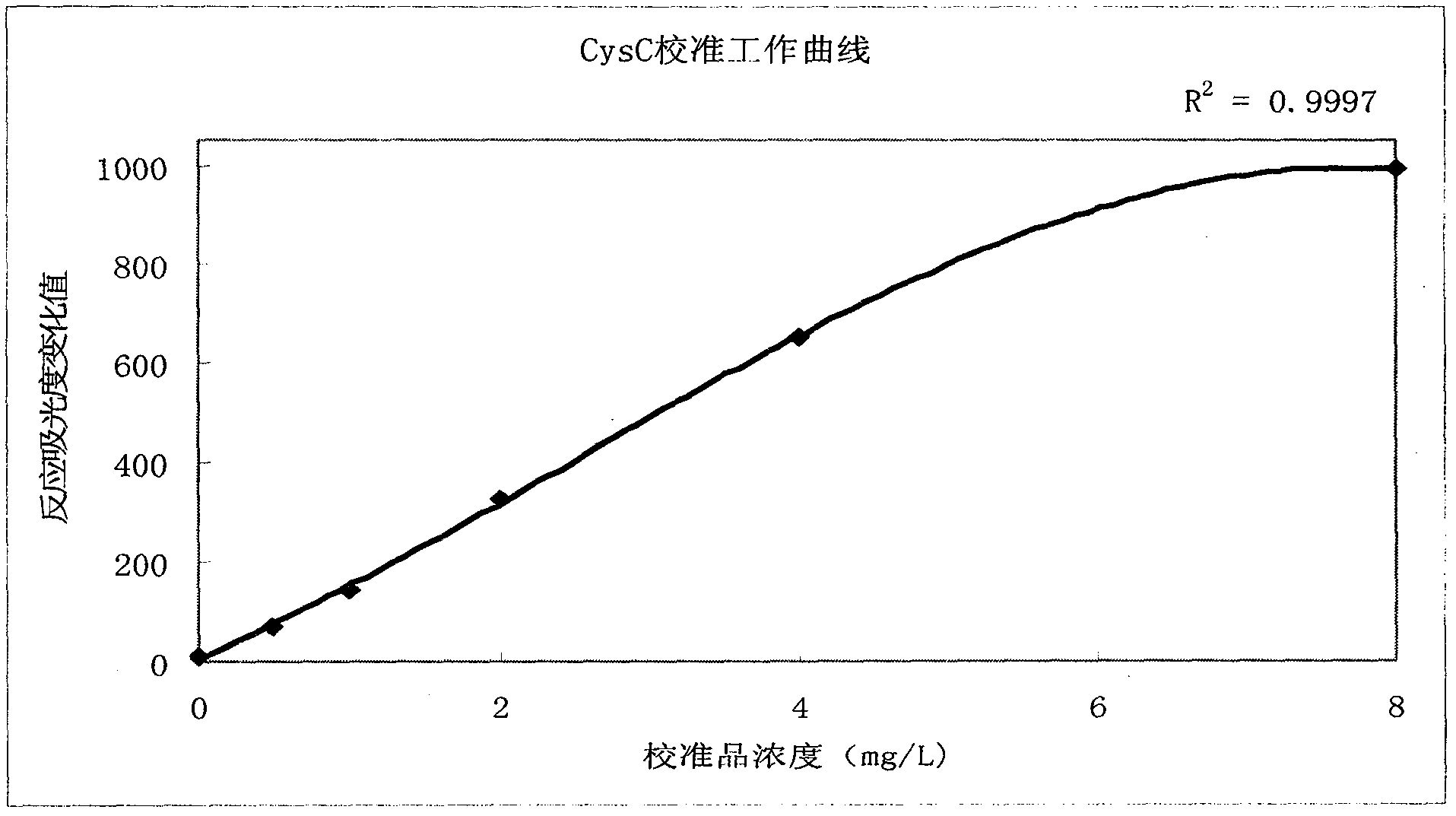
[0038] (1) First use the Mindray BS300 automatic biochemical analyzer to calibrate according to the method steps mentioned in the kit manual, and the working curve is shown in the appendix figure 2 .
[0039] (2) Dilute the purified protein to about 8.50 μg / ml with protein protection solution, and use the above curve to measure three times, and the average value of the result is 7.80 μg / ml.
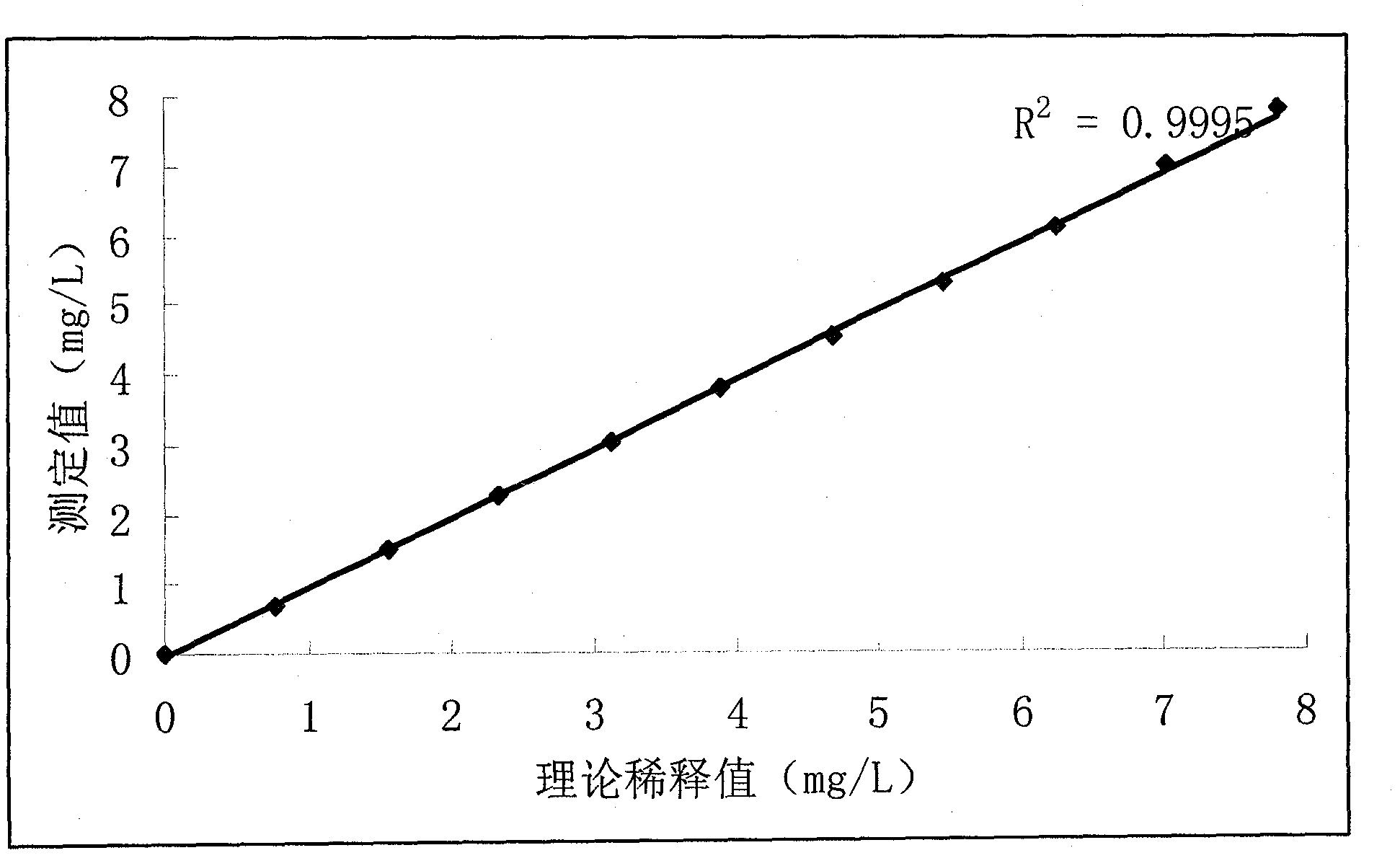
[0040] (3) Use the protein protection solution to dilute the above-measured protein ratio to a series of values (including the original concentration and 0 concentration of the protein protection solution), that is, 7.80 μg / ml, 7.02 μg / ml, 6.24 μg / ml, 5.46 μg / ml ml, 4.68 μg / ml, 3.90 μg / ml, 3.12 μg / ml, 2.34 μg / ml, 1.56 μg / ml, 0.78 μg / ml, 0 μg / ml.
[0041] (4) Then use a biochemical analyzer to measure the above concentration values three times to get the average value, and the correlation coeffic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com