Quantitative immune colloidal gold detection card and kit for urocystatin C, urine microalbumin and urine creatinine
A technology of urinary microalbumin and colloidal gold, which is applied in the field of colloidal gold immunochromatography, can solve the problems of complex operation of the detection method, long detection time, high detection cost, etc., and achieve improved detection rate and detection accuracy, simple operation, The effect of simplifying inspection operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
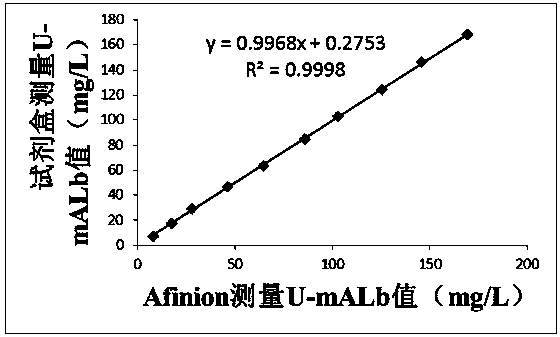
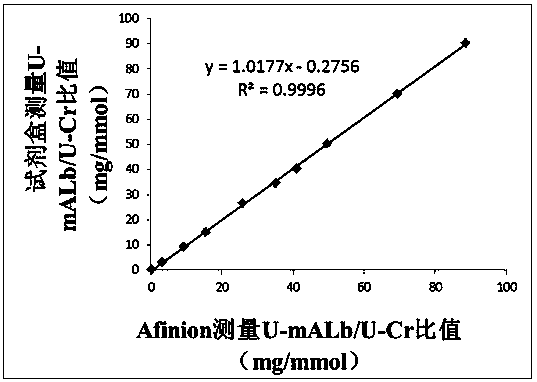
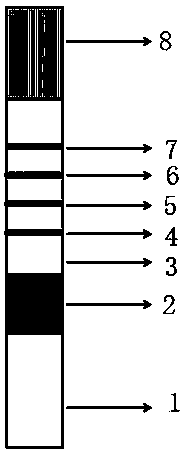
[0050] like Figure 1~2 As shown, this embodiment provides a quantitative immune colloidal gold detection card for urinary cystatin C, urinary microalbumin and urine creatinine, including a snap box, a base plate, and a sample pad, a binding pad, and a chromatography pad sequentially pasted on the base plate. Membrane and sample suction pad, the bottom plate is installed in the buckle box; wherein, the bottom plate is PVC board, the chromatography membrane is nitrocellulose membrane, the sample suction pad is water-absorbing filter paper, and the sample pad and binding pad are made of glass fiber.
[0051] In this example, colloidal gold-labeled cystatin C antibody I, urine microalbumin antibody I, urine creatinine antibody I and chicken IgY are coated on the binding pad, and the chromatographic membrane is coated with cystatin C antibody II The first detection line, the second detection line coated with urinary microalbumin antibody II, the third detection line coated with BS...
Embodiment 2
[0059] The present embodiment provides a preparation method of urinary cystatin C, urine microalbumin and urine creatinine quantitative immune colloidal gold detection card, specifically comprising the following steps:
[0060] S1. Preparation of colloidal gold labeled antibody application solution: first prepare colloidal gold solution, put 1L ultrapure water in the reactor, add chloroauric acid to it, add trisodium citrate after boiling, continue to boil for 10 minutes, chloroauric acid is finally The concentration is 0.04%, and the final concentration of trisodium citrate is 0.01%. After cooling, dilute to 1L with ultrapure water, collect the product, and the diameter of the colloidal gold solution is 40mm;
[0061] Take 5 mL of colloidal gold solution, under slow stirring, adjust the pH value of the solution to 7.5 with 0.2 M potassium carbonate buffer solution, then add 30 μg Cystatin C antibody I, continue stirring for 30 min, add BSA with a final concentration of 0.5%, ...
Embodiment 3
[0073] The present embodiment provides a preparation method of urinary cystatin C, urine microalbumin and urine creatinine quantitative immune colloidal gold detection card, specifically comprising the following steps:
[0074] S1. Preparation of colloidal gold labeled antibody application solution: first prepare colloidal gold solution, put 1L ultrapure water in the reactor, add chloroauric acid to it, add trisodium citrate after boiling, continue to boil for 10 minutes, chloroauric acid is finally The concentration is 0.05%, and the final concentration of trisodium citrate is 0.02%. After cooling, dilute to 1L with ultrapure water, collect the product, and the diameter of the colloidal gold solution is 45mm;
[0075] Take 5 mL of colloidal gold solution, under slow stirring, adjust the pH value of the solution to 7.5 with 0.2 M potassium carbonate buffer solution, then add 20 μg Cystatin C antibody I, continue stirring for 30 min, add BSA with a final concentration of 0.5%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com