Magnetic particle separation chemiluminescence immunoassay method of human cystatin C
A chemiluminescence immunoassay and detection method technology, applied in the field of magnetic separation chemiluminescence immunoassay of human cystatin C, which can solve the problems of poor sensitivity, precision and specificity
Inactive Publication Date: 2011-01-05
北京倍爱康生物技术有限公司
View PDF4 Cites 25 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, the Cystatin C kit used in clinical testing mainly adopts immunoturbidimetric method, which is obviously inferior in sensitivity, precision and specificity compared with enzyme-linked immunoassay, immunofluorescence technology, chemiluminescence immunoassay and other analytical techniques.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Login to View More
Abstract
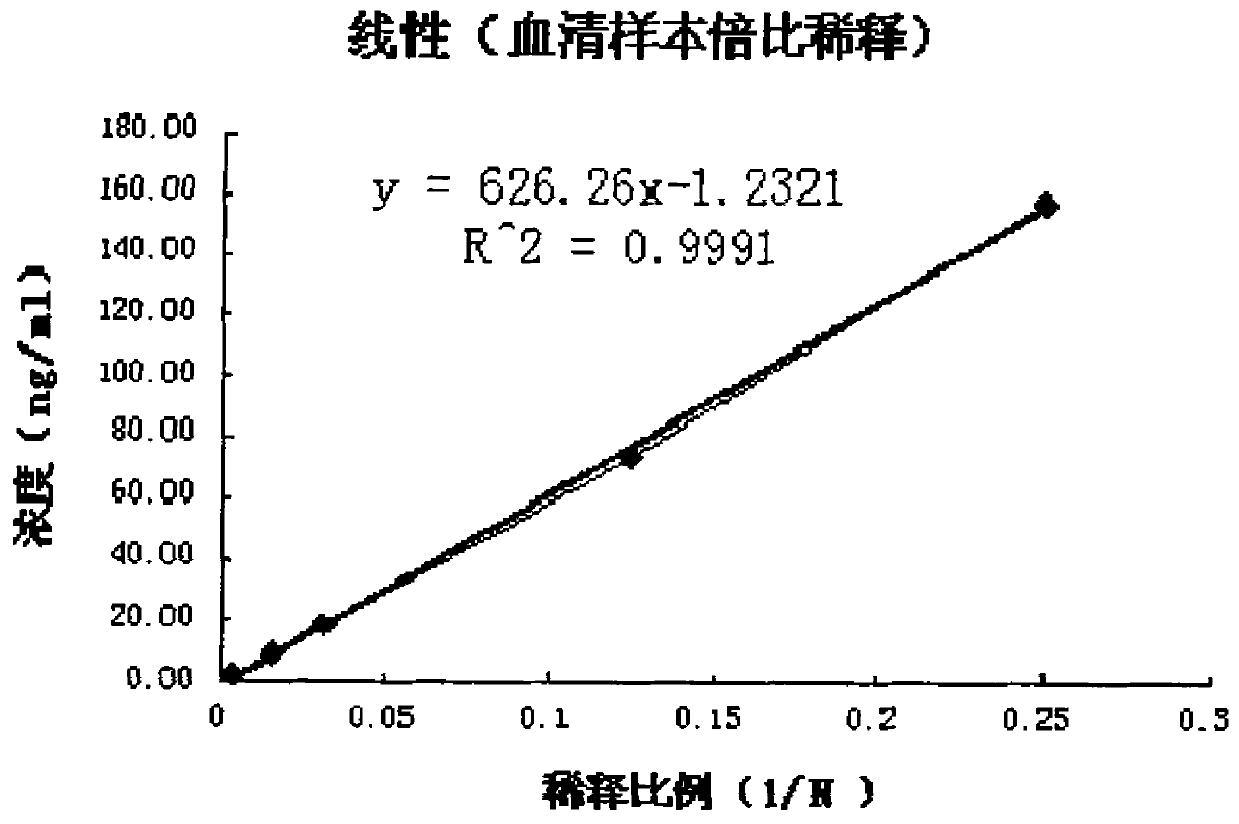
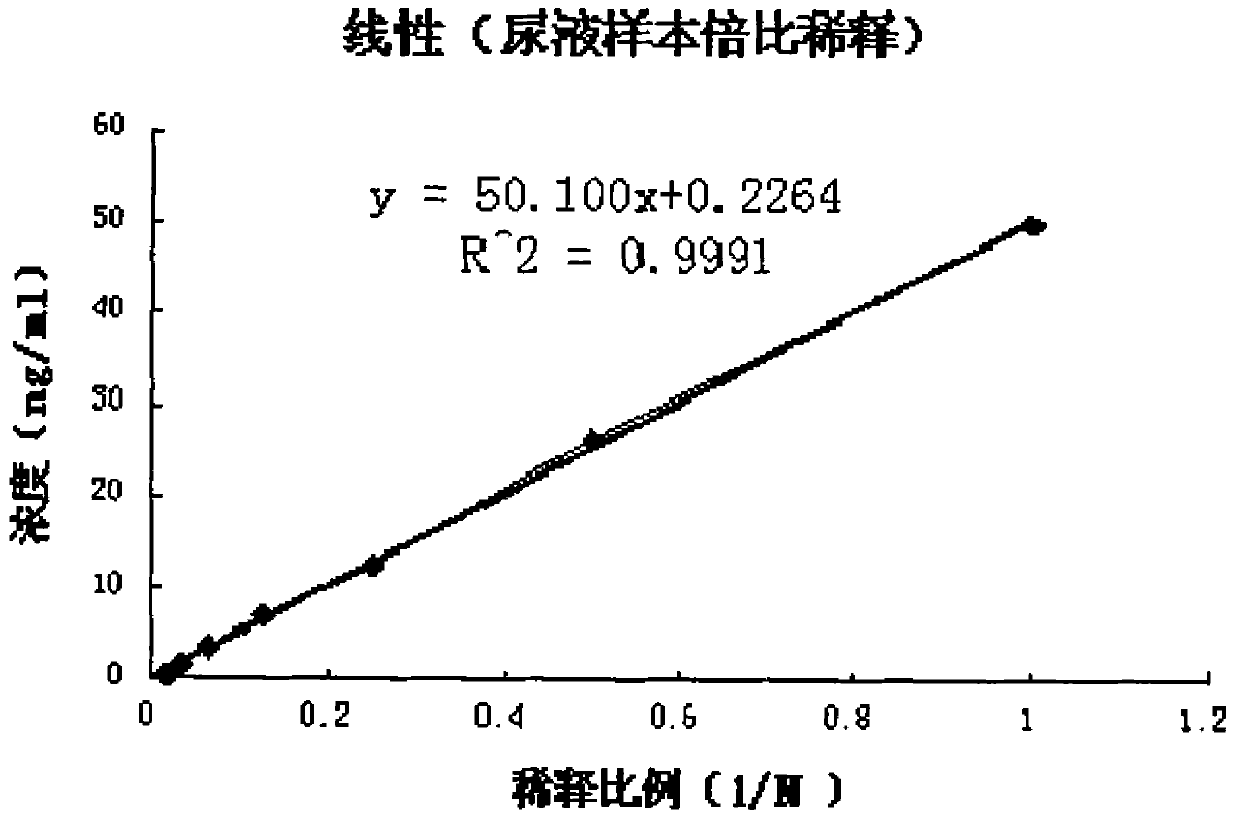
The invention provides an in-vitro detection method of human cystatin C. The method adopts a magnetic particle separation chemiluminescence immunoassay technology which is a product integrating an enzyme labeling technology, a magnetic particle separation technology and a chemiluminescence detection technology and has the advantages of high sensitivity, good specificity, good repetitiveness, and the like. The method can be used for measuring the content of cystatin C in the serum, the plasma and the urine of a person, and the indexes are mainly used for clinically evaluating the renal function and mainly used for monitoring the filtration rate of glomerulus, the renal tubular dysfunction and various secondary nephropathies.
Description
A magnetic particle separation chemiluminescence immunoassay detection method for human cystatin C technical field The invention belongs to the technical field of immunodetection and analysis, relates to a diagnostic index cystatin C for clinically evaluating renal function, and provides a magnetic separation chemiluminescence immunoassay method for human cystatin C, which is applicable to human serum and plasma Or quantitative detection of cystatin C in urine. Background technique Cystatin C (Cystatin C) is a member of the cysteine protease inhibitor family. It is a non-glycosylated basic protein with a relative molecular mass of 13.395 Da and an isoelectric point of 8.0-9.5. Composed of 122 amino acids. The cystatin C coding gene is located on human chromosome 20 and has the same characteristics as the promoter region of the "housekeeping gene", so the cystatin gene is regarded as a "housekeeping gene". Cystatin C widely exists in plants, animals and protozoa. It is ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): G01N33/68G01N21/76G01N33/535
Inventor 郭健夫张雪莲鲁衡仝文斌
Owner 北京倍爱康生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com