Recombinant human cystatin C coding gene and expression method
An expression method and coding gene technology, which are applied in the field of recombinant human cystatin C coding gene and expression, can solve the problems of hindering popularization and application, limited antibody sources, low expression efficiency, etc., and meet the limitations of preparation conditions and selection of vector types. less, the construction process is simple, and the expression efficiency is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The expression method of recombinant human cystatin C protein comprises the following steps:
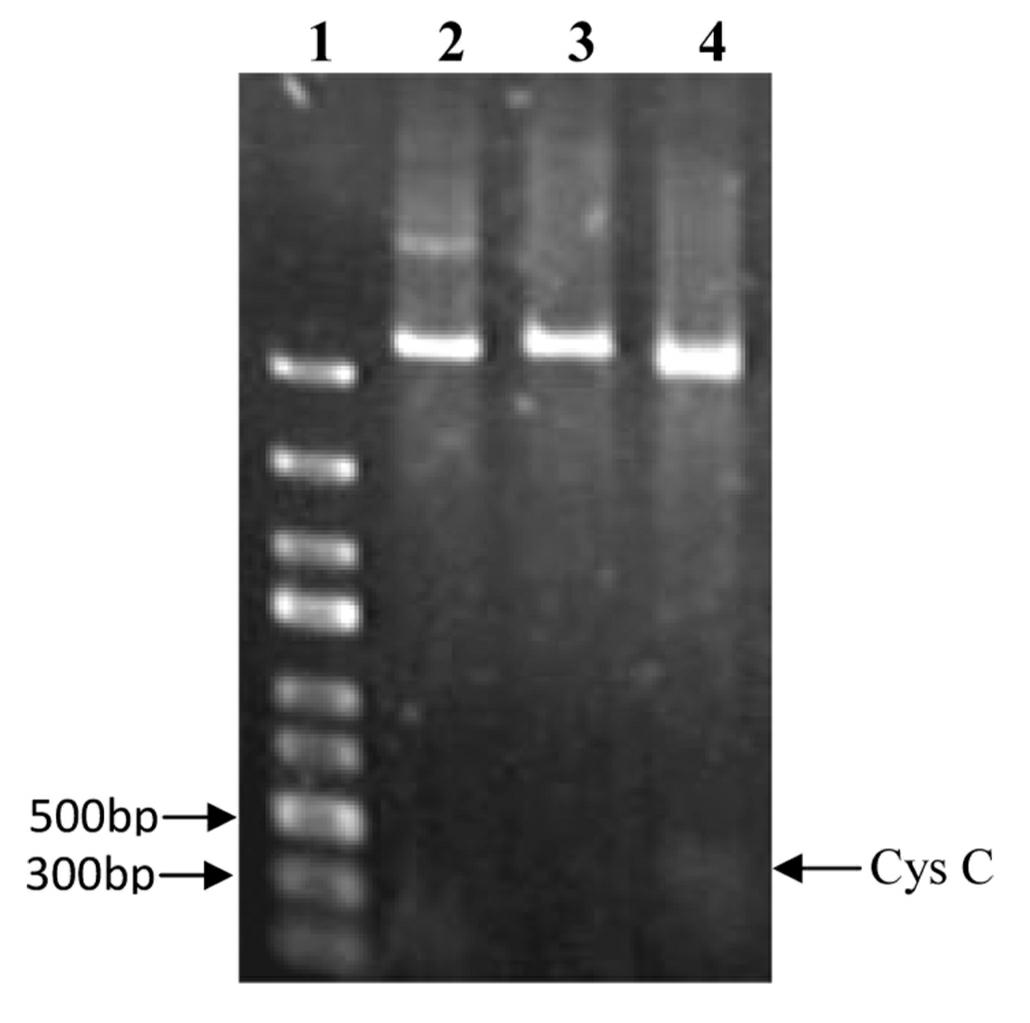
[0035] 1) Add NdeI restriction site (catatg) to the 5' end of the target gene (nucleotide sequence is SEQ ID NO: 1), and EcoRI restriction site (gaattc) to the 3' end to synthesize the entire sequence to obtain Synthetic gene sequences;
[0036] 2) The synthetic gene sequence was cloned into the pMD18-T vector, and sequenced to confirm the successful construction of the recombinant T vector;
[0037] 3) The target gene sequenced correctly was excised from the recombinant T vector with NdeI and EcoRI endonucleases, and purified to obtain the target gene. The prokaryotic expression vector pET-22b(+) was subjected to the same enzyme digestion treatment, and the large fragment gene was purified. Then connect with the target gene obtained after double enzyme digestion, transfer into competent cell DH5α, extract the plasmid, and obtain the recombinant expression plasmid pET-CysC; ...
Embodiment 2
[0042] The expression method of recombinant human cystatin C protein comprises the following steps:
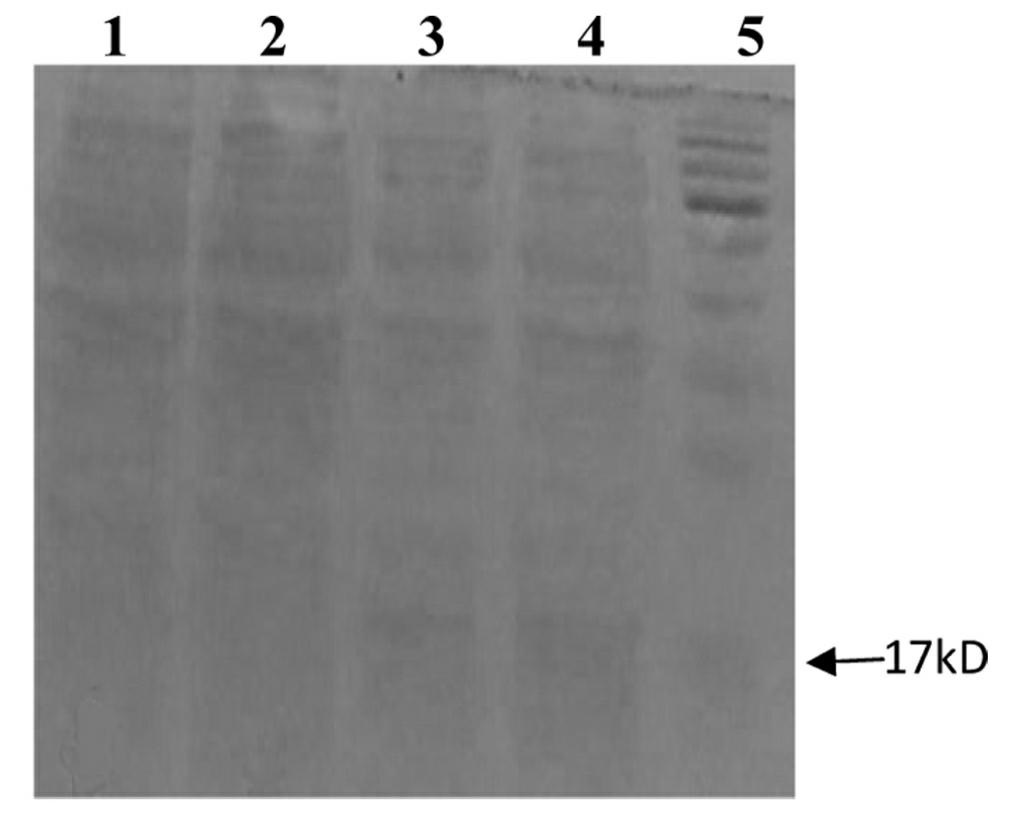
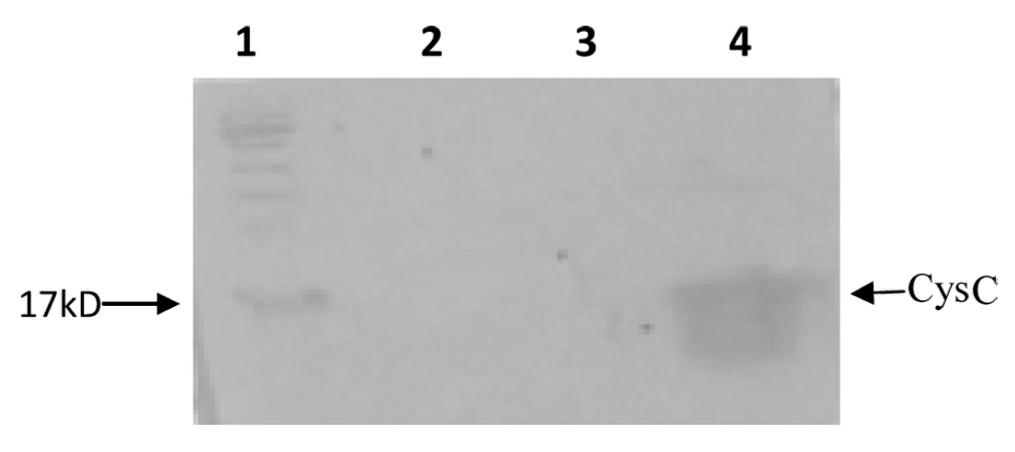
[0043]1) Transfer the recombinant expression plasmid pET-Cys C obtained in step 3) of Example 1 into the competent expression host strain E.coli BL21(DE3), and spread it on LB solid medium (containing 100 mg / L ampicillin) above, cultured at 37°C for 16 hours, and the positive clones were screened out as engineering bacteria;
[0044] 2) Inoculate the engineered bacteria into LB culture solution (containing 100 mg / L ampicillin), culture at 37°C, and wait until the OD of the bacteria solution 600nm At 0.6, add IPTG with a final concentration of 0.1 mmol / L, incubate at 37°C for 10 h, and centrifuge at 4°C to obtain bacterial cells;
[0045] 3) Same as Step 6 of Example 1).
Embodiment 3
[0047] The expression method of recombinant human cystatin C protein comprises the following steps:
[0048] 1) Transfer the recombinant expression plasmid pET-Cys C obtained in step 3) of Example 1 into the competent expression host strain E.coli BL21(DE3), and spread it on LB solid medium (containing 100 mg / L ampicillin) above, cultured at 37°C for 24 hours, and the positive clones were screened out as engineering bacteria;
[0049] 2) Inoculate the engineered bacteria into LB culture solution (containing 100 mg / L ampicillin), culture at 37°C, and wait until the OD of the bacteria solution 600nm At 1.0, add IPTG with a final concentration of 10 mmol / L, incubate at 37°C for 2 h, and centrifuge at 4°C to obtain bacterial cells;
[0050] 3) Same as Step 6 of Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com