Cystatin C detection reagent and method
A technology for detecting reagents and cystatin, which is applied in the field of cystatin C detection reagents, can solve problems such as lack of anti-interference ability, achieve good stability of reagents, high accuracy, and improve work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: The present invention detects the reagent and method of cystatin C
[0072] The reagents for detecting cystatin C in this embodiment include:
[0073] Reagent 1: ammonium chloride 0.2mol / L, sodium azide 0.05%, Brij-35 0.3%, sodium chloride 150mmol / L, PEG6000 2%, adjust the pH value with ammonia water, pH 7.5;
[0074] Reagent 2: Antibody-coated latex particles (0.1% concentration), Tris-Hcl buffer 50mmol / L, sucrose 3%, gelatin 0.5%, NP30 0.1%, Proclin300 0.3%, pH7.0.
[0075] Wherein, the preparation method of the latex particle coated with antibody is:
[0076] (1) Take 1ml (10mg / mL) of the washed latex particles, add it to 50mL of ethylenediamine (EDA, 1mol / L, pH4.75), and incubate for 90min.
[0077] (2) Add solid EDC to a final concentration of 10 mM, and stir slowly for 90 min.
[0078] (3) Wash thoroughly with pure water, and resuspend in pH7.5 phosphate buffer for the last time.
[0079] (4) Latex particles (99nm in diameter) with an antibody co...
Embodiment 2
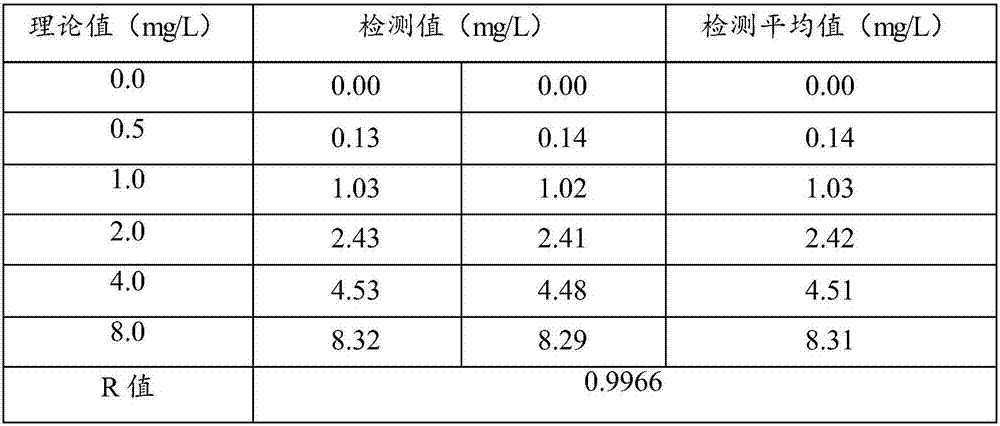
[0082] Embodiment 2: the accuracy analysis of reagent of the present invention and method
[0083] Test instrument: Hitachi 7080 automatic biochemical analyzer
[0084] Test samples: blood samples from 20 medical examiners;
[0085] Comparative method kit: commercially available domestic cystatin C kit (latex-enhanced immune turbidimetric method);
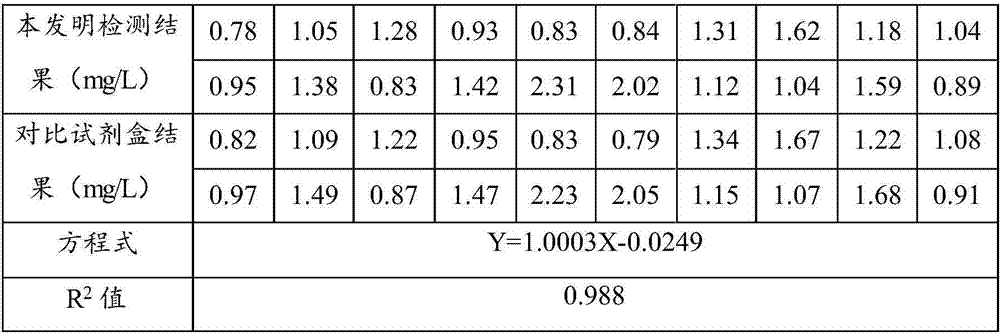
[0086] 20 examples of samples were measured respectively with the detection method of Example 1 and the comparison method, and the detection results are shown in Table 1.
[0087] Table 1 analysis results
[0088]
[0089] The results show that the R calculated according to the test results 2 The value is 0.988, which is greater than 0.95, showing that the detection result of the method of the present invention has no obvious difference with the detection result of the comparison kit, and has a relatively high degree of accuracy (degree of coincidence).
Embodiment 3
[0090] Embodiment 3: the precision analysis of reagent of the present invention and method
[0091] Test instrument: Hitachi 7080 automatic biochemical analyzer;
[0092] Test sample: any serum sample:
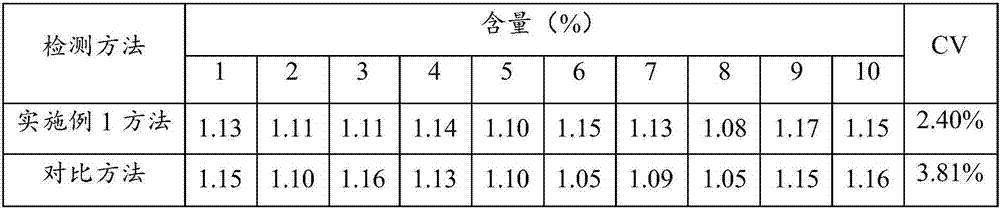
[0093] Using the detection method of Example 1 and the detection method of Example 3 of the comparative patent (CN201410735830.9 - an improved cystatin C detection kit) to repeat the detection 10 times on the same sample to be tested, the detection results are shown in Table 2.
[0094] Table 2 detects sample cystatin C concentration (10 times) and standard deviation rate (variation coefficient)
[0095]
[0096] The results show that the standard deviation rate of the present invention is 2.4%, less than 10% of the standard requirement, and less than 3.81% of the comparative patent method, indicating that the method of the present invention has relatively high precision.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com