A markerless gene deletion attenuated mutant strain of Vibrio alginolyticus wild strain, related preparations and applications
A marker-free, alginolytic vibrio technology, applied in microorganism-based methods, medical preparations containing active ingredients, bacteria, etc., can solve the problem that the immune effect is not as good as that of live vaccines, the immune dose and cost are high, and it is not infectious. and other problems, to achieve the effect of eliminating the possibility of toxic pathogens, significant immune effect, and good control effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
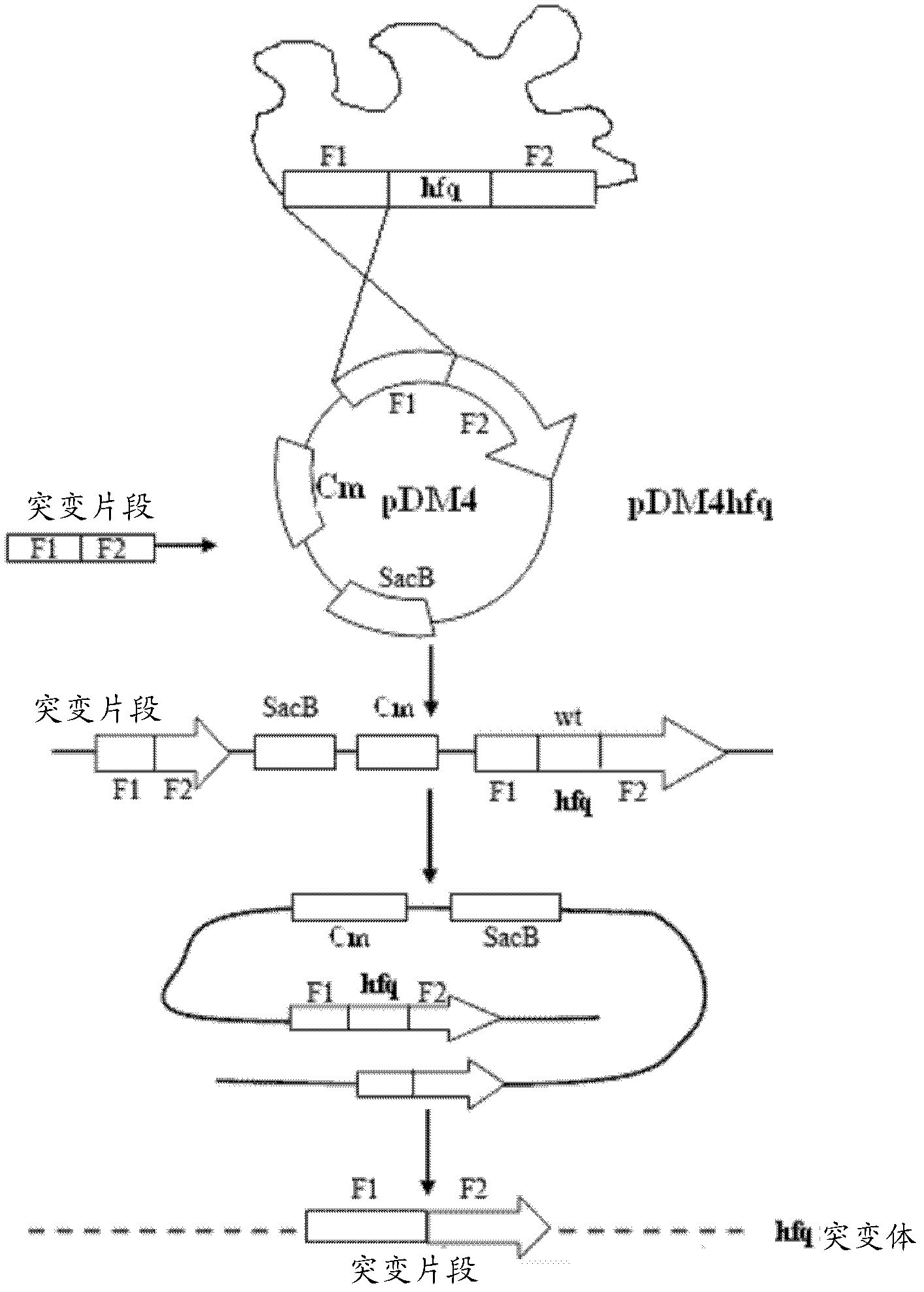
[0030] Example 1 Construction of Markerless Gene Deletion Attenuated Mutants
[0031] (1) Construction of hfq gene deletion strain
[0032] 1) PCR amplification to obtain the desired gene fragment
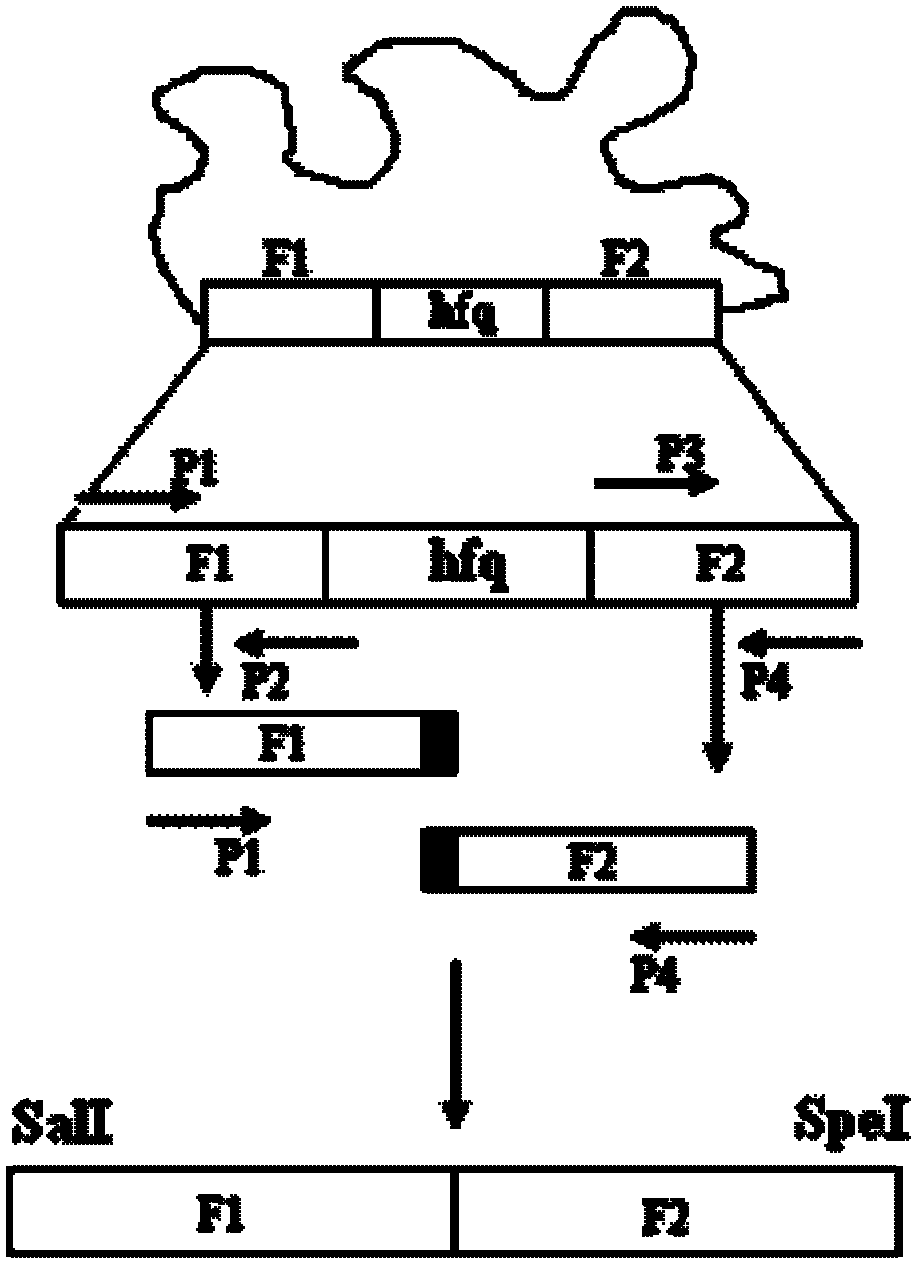
[0033] Such as figure 1 As shown, the genome of Vibrio alginolyticus strain EPGS020401 (preservation number: CCTCC NO: AB 209306) is used as a template, and the following amplification primers are used:
[0034] P1(TTAGTCGACCCGACAGATGTGGGAGTATTTAGAT),
[0035] P2(TGTGGACGCTCGTCTTGTAGAGATTGCCCCTTAGCC),
[0036] P3(CTCTACAAGACGAGCGTCCACAAAGAGAAATCTGAAG),
[0037] P4(GCTACTAGTGATATCGAGAATAAGACCAGTGCGG),
[0038] First, use P1 and P2, P3 and P4 to amplify the upper and lower fragments F1 and F2 required by Overlap PCR respectively. After each fragment was recovered, the hfq deletion fragment F1F2 was obtained by using P1 and P4 using the Overlap PCR technique.
[0039] 2) Recover each fragment
[0040] To recover the target gene fragment from the object to be studied, use the g...
Embodiment 2
[0056] Embodiment 2: Taking zebrafish (Danio rerio) as the semi-lethal dose LD of experimental animals 50 Determination:
[0057] The fish used in the experiment were first placed in the SPF (Specific Pathogen Free) laboratory to adapt to breeding for 1 week to remove abnormal individuals. Before the infection test, the SPF test fish were stocked in a 0.5L infection test tank in the Challenge Lab, and continued to be fed for 1 week, with 10 fish (average body length 2.5-3cm, body weight 0.2g) in each tank. The test tank replaced 1 / 2 volume of culture water with sterile old water every day, and the water temperature was 28°C, with a fluctuation of 2°C.
[0058] The fish used in the test were randomly divided into groups, and two tanks were tested in parallel in each group. In the infection test, each group of test fish was treated with a certain gradient dose (10 2 -10 7 CFU / tail) of Vibrio alginolyticus wild strain and attenuated vaccine strain were artificially infected b...
Embodiment 3
[0063] Embodiment 3: Taking zebrafish as the experimental animal's immune protection test by injection
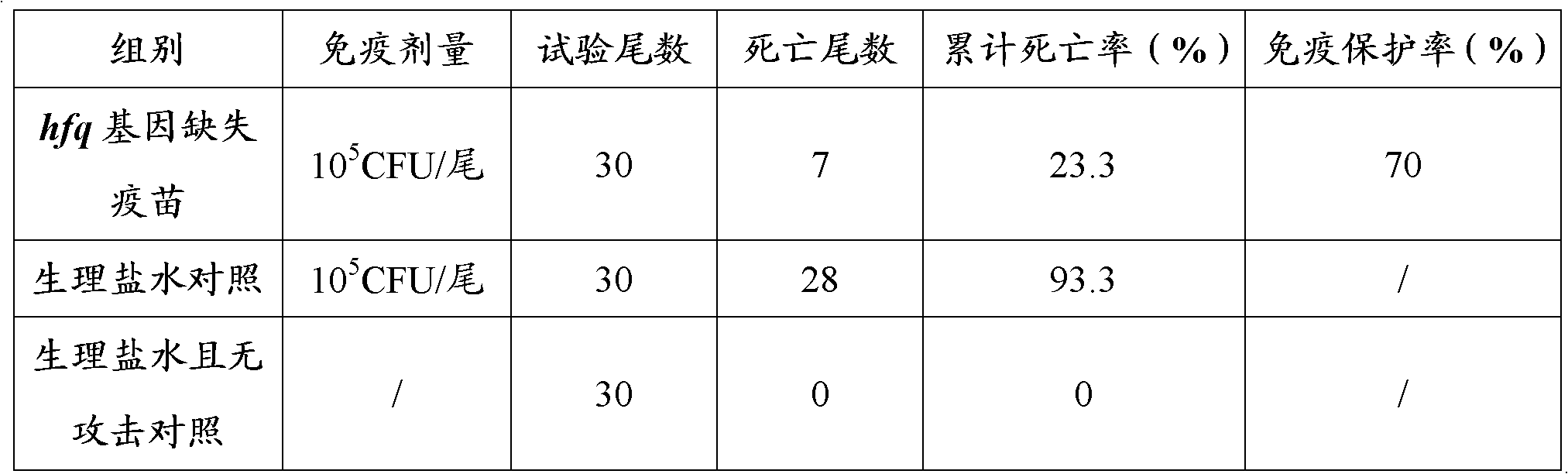
[0064] The experimental zebrafish were randomly divided into 3 groups, each with 3 parallel tanks, 10 fish per tank. The prepared attenuated live vaccine was immunized by intramuscular injection. Injection immunization dose is 7×10 5 CFU / g, intramuscular injection test zebrafish. The control group was injected with sterile normal saline. After 4 weeks of immunization, the zebrafish of each group were infected with live bacteria of the wild strain of Vibrio alginolyticus (intramuscular injection of 5×10 5 CFU / tail) for artificial infection challenge. Observe and count the control group and the number of immune deaths within 15 days to calculate the immune protection rate of each group (see Table 2).
[0065] Wherein, the immune protection rate was calculated according to the following formula: immune protection rate%=(control group death rate−immune group death rate%) / c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com