Diploid-cell rabies vaccine and purified rabies vaccine, freeze-drying preparation and water injection thereof
A technology of diploid cell and rabies vaccine, applied in the field of rabies purified vaccine, can solve the problem that exogenous factors cannot guarantee, cannot guarantee tumorigenicity of cell matrix and cell DNA, cannot guarantee that cells do not carry, etc., and achieve good antibody level , the effect of improving stability and increasing durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
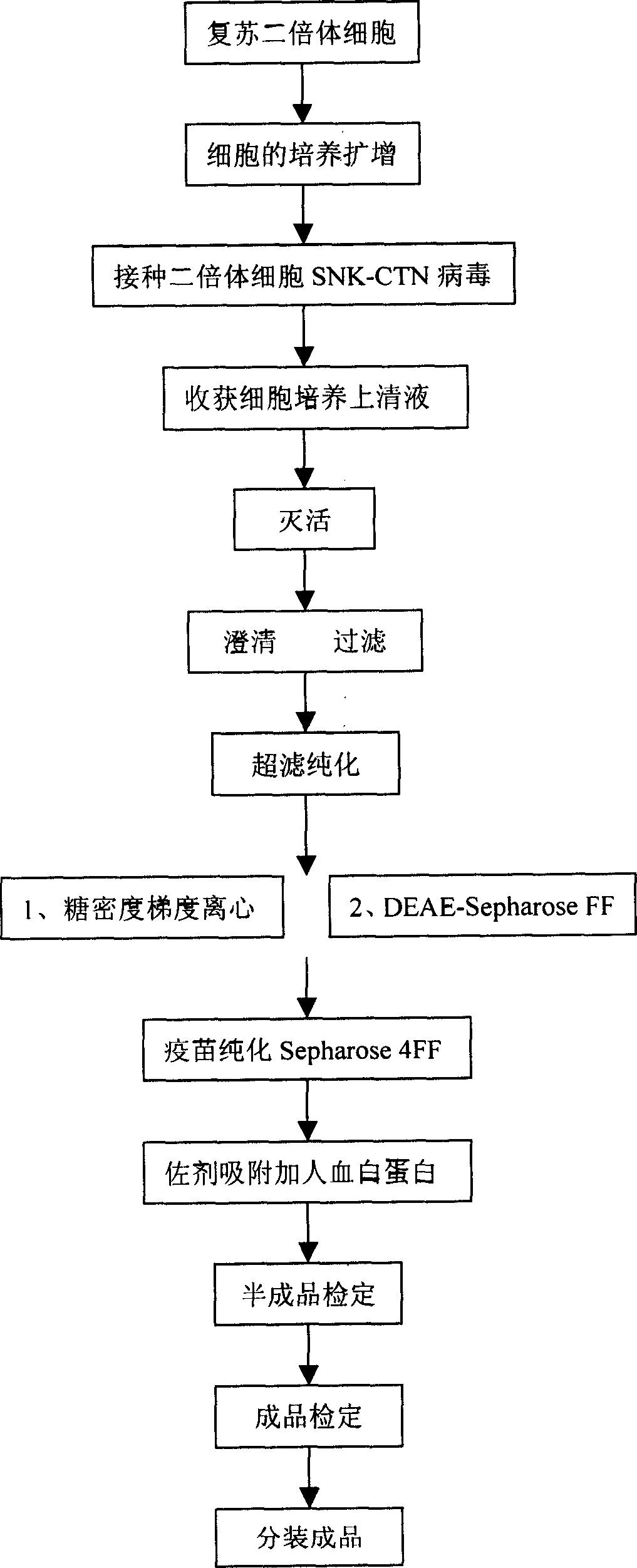
[0055] Diploid cells come from the Chinese Center for Disease Control and Prevention, and the cell nutrient solution is 199 medium containing 2-10% bovine serum, 25IU / ml gentamycin and 25IU / ml kanamycin, and adjust the pH to 7.2 with The 3L spinner bottle was cultured at 37°C to form a dense monolayer and then amplified according to the seeding ratio of 1:2. When it was expanded to the production batch, after about 4 days, when the cells grew into a dense monolayer, the cell nutrient solution was discarded, and the pH7. Wash the cell surface with 2 Earl's solution, inoculate the diploid cell rabies virus, use the second generation working virus of the rabies virus SNK-CTN strain passed on the diploid cells, the concentration of the virus is MOI0.05, and the cell maintenance solution is 199 culture medium containing 0.4-0.5% (W / W) human serum albumin, pH 7.6, cultivate at 35°C, replace with fresh cell maintenance medium after 24 hours and continue to culture for 2 days, start to...
Embodiment 2
[0059] Diploid cells come from the Chinese Center for Disease Control and Prevention, and the cell nutrient solution is 199 medium containing 2-10% bovine serum, 25IU / ml gentamycin and 25IU / ml kanamycin, and adjust the pH to 7.2 with After cultured in a 3L spinner bottle at 37°C to form a dense monolayer, amplify according to the seeding ratio of 1:2. When the expansion reaches the production batch, after about 4 days, when the cells grow into a dense monolayer, the cell nutrient solution is discarded, and the pH7. Wash the cell surface with 2 Earl's solution, inoculate the diploid cell rabies virus, use the second generation working virus of the rabies virus SNK-CTN strain passed on the diploid cells, the concentration of the virus is MOI0.05, and the cell maintenance solution is 199 culture medium containing 0.4-0.5% (W / W) human albumin, pH 7.6, cultivate at 35°C, replace with fresh cell maintenance medium after 24 hours and continue to culture for 2 days, start to harvest vi...
Embodiment 3
[0063] With the inactivated rabies vaccine prepared by the method of claim 1, after 14 days of inoculating the purified rabies vaccine to those who have not been vaccinated against rabies, the positive conversion rate of neutralizing antibodies is higher than 95.0%, and no side effects occur.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com