Cpg Single Strand Deoxynucleotides for Use as Adjuvant
a single-strand deoxynucleotide and adjuvant technology, applied in the field of single-strand deoxynucleotide containing cpg dinucleotides, can solve the problems of not thoroughly eliminating latent hbvs inside the infected cells, no effective treatment of rabies infections, and severe effects on the health of people around the globe, so as to improve the immune response of the body, enhance the immunogenicity of the hbv vaccin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of the Single Strand Deoxynucleotide Containing CpG
[0041] The sequences were designed as follows:
(1) (G)n(L)n X1X2CGY1Y2(M)n (G)nX1 = A, T, G; X2 = A, T; Y1 = A, T; Y2 = A, T, C; L, M = A, T,C, G; n is 0-6.5′-ggggTCgTTCgTCgTTgggggg-3′(SEQ ID NO: 1)[121]5′-ggggATAACgTTgCgggggg-3′(SEQ ID NO: 2)[143]5′-ggggTgCAACgTTCAgggggg-3′(SEQ ID NO: 3)[402]5′-ggggTCCTACgTAggAgggggg-3′(SEQ ID NO: 4)[123]5′-ggggTCCATgACgTTCCTgAAgggggg-3′(SEQ ID NO: 5)[603]5′-gggggACgTCgCCggggggg-3′(SEQ ID NO: 6)[118]5′-ggATCCgTACgCATgggggg-3′(SEQ ID NO: 7)[320]5′-gggggAATCgATTCgggggg-3′(SEQ ID NO: 8)[154]5′-gggATgCATCgATgCATCgggggg-3′(SEQ ID NO: 9)[464]5′-ggTgCgACgTCgCAgggggg-3′(SEQ ID NO: 10)[471]5′-gggACgTACgTCgggggg-3′(SEQ ID NO: 11)[390]5′-gggggATCgACgTCgATCgggggg-3′(SEQ ID NO: 12)[322]5′-ggCgATCgATCgATCggggggg-3′(SEQ ID NO: 13)[333]5′-ggggTCgATCgATCgAgggggg-3′(SEQ ID NO: 14)[113]5′-ggTCgCgATCgCgAgggggg-3′(SEQ ID NO: 15)[307]5′-ggGGTCAACGTTGAgggggG-3′(SEQ ID NO: 16)[156]5′-gTCgTTTTCgTCgACgAATTgggggggg-...
example 2
Synthesis of the Single Strand Deoxynucleotide Containing CpG
[0044] DNA fragment was synthesized by solid phasephosphoramidite triestermethod. The method has been widely used in chemical synthesis of DNA due to its advantages of fast, high efficiency, etc.
[0045] Chemical synthesis of DNA is different from enzymatic synthesis of DNA. The latter is synthesized from 5′ end to 3′ end, while the former starts from 3′ end. The synthesis steps are as follows:
[0046] 1. De-Protection
[0047] Trichloroacetic acid was used to remove protective group DMT conjugated to the nucleotides on Controlled Pore Glass (CPG) to obtain free 5′ hydroxyl ends for being used in the following condensation reaction.
[0048] 2. Activation
[0049] The nucleotides units protected by phosphoramidite was mixed with tetrazolium activator and then loaded into a synthesis column to form an active intermediate of phosphoramiditetetrazole (the 3′ end of which is activated, while the 5′ end of which is still under DMT pro...
example 3
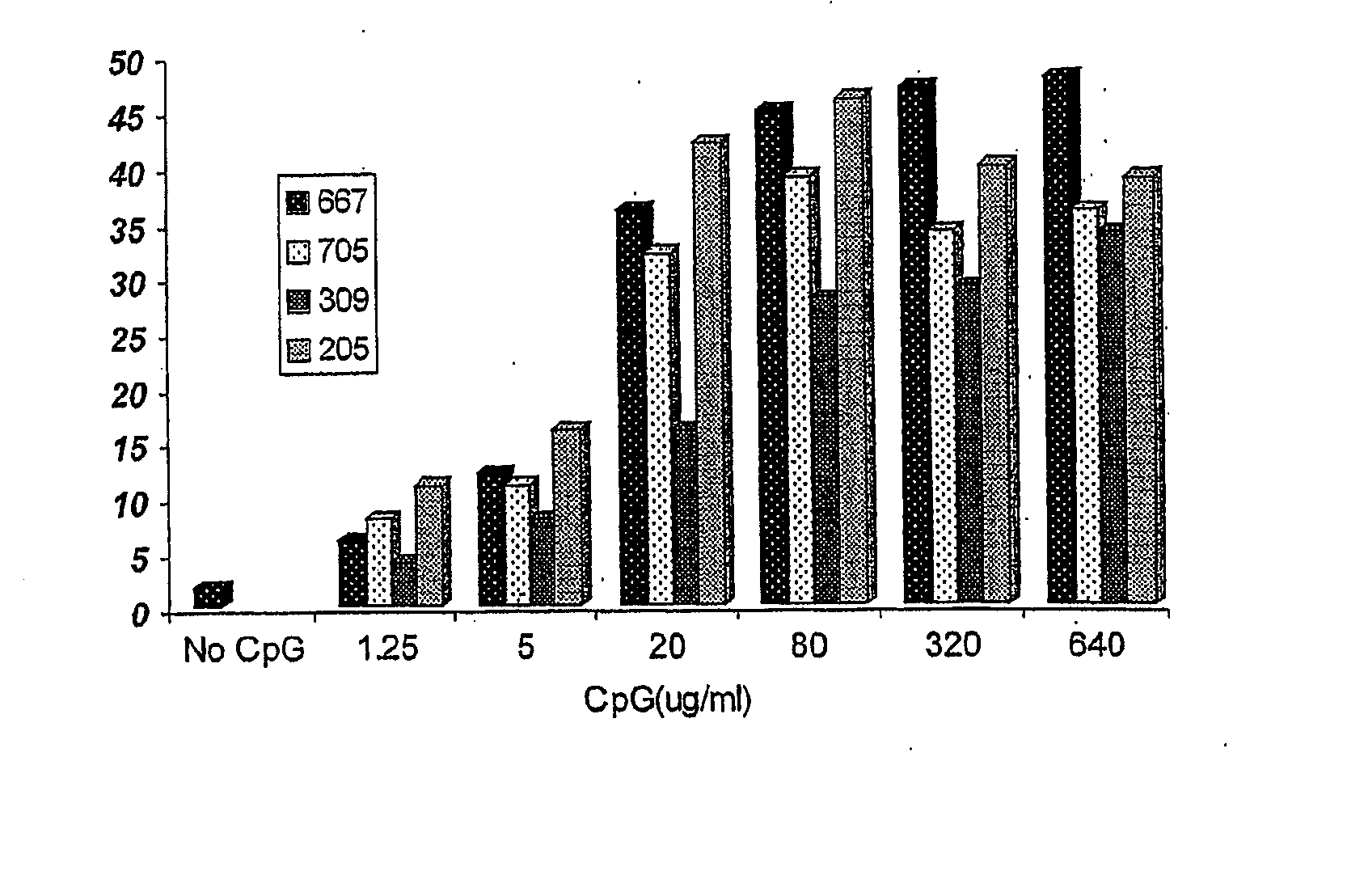
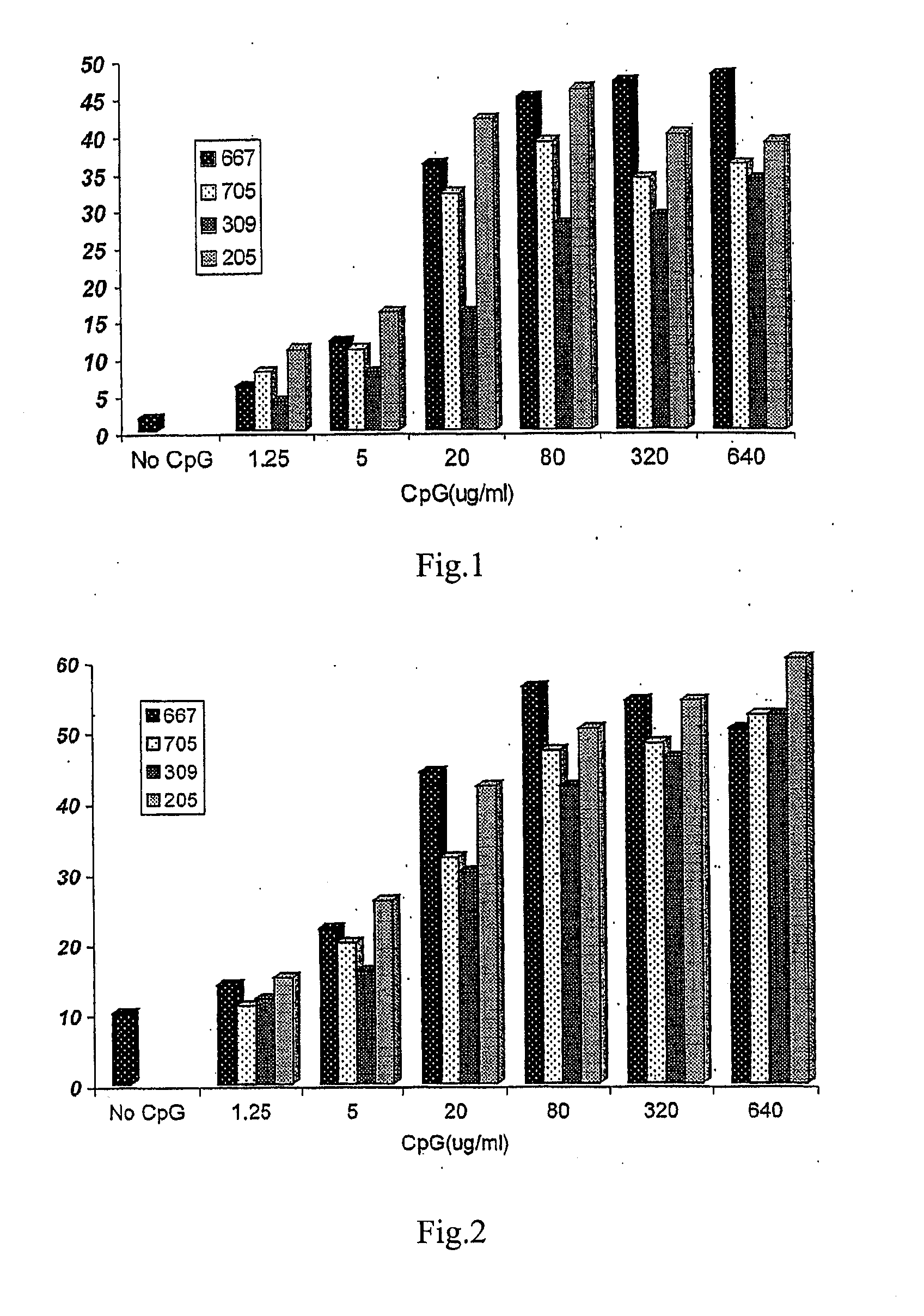
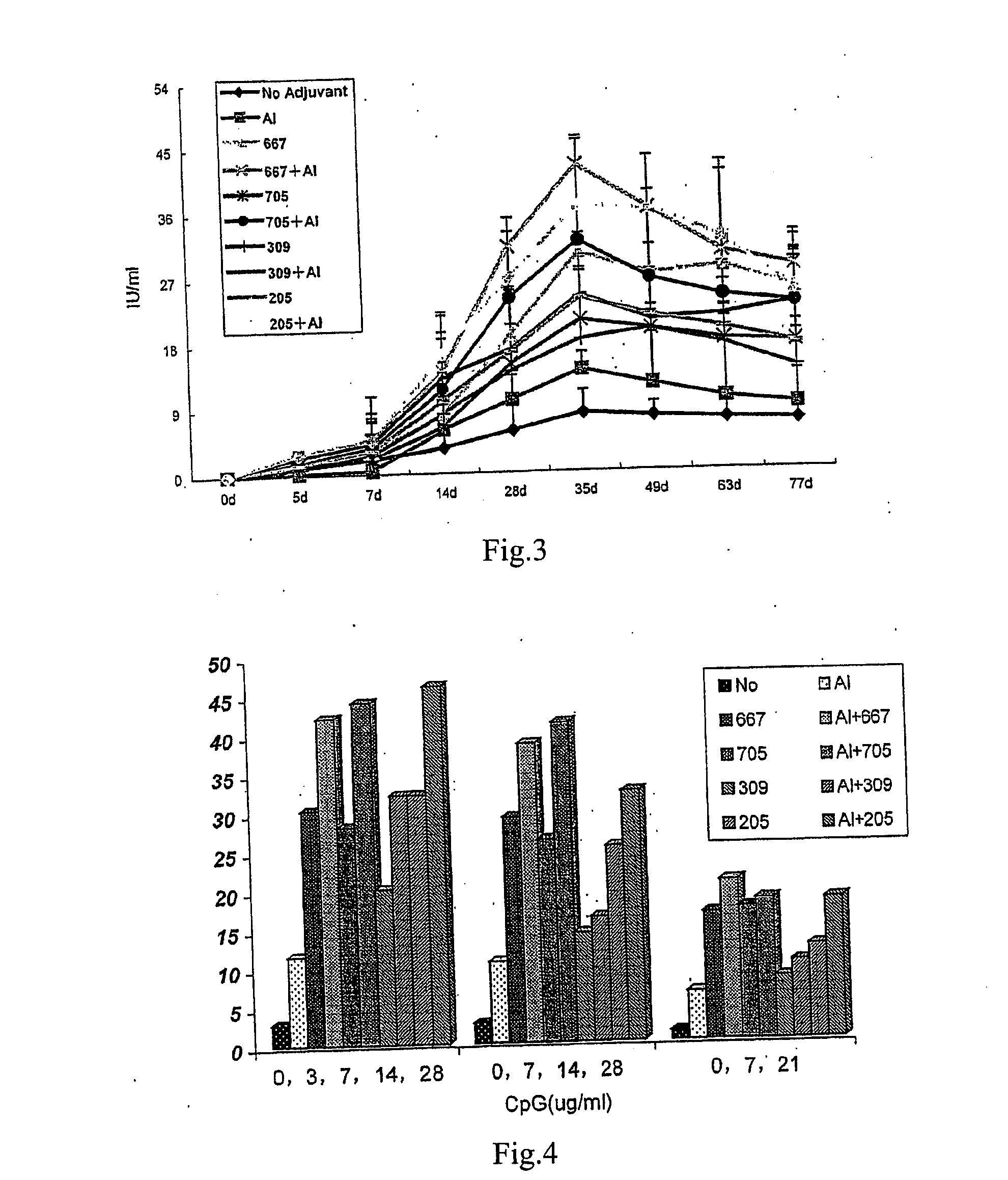
Effects of Different Dosages of a CpG ODN on Antibody Production Stimulated by Rabies Vaccine
[0059] 1. Experimental animals: 200 white mice, with half males and half females, weighted from 18 to 22 g, aged from 6 to 8 weeks, and purchased from Beijing Weitonglihua Experimental Animal Ltd.
[0060] 2. Rabies vaccine: 1 ml / vial (containing 2.5 IU), purchased from Changchun Institute of Biological Product.
[0061] 3. CpGODN: synthesized by Shanghai Shenggong Biotechnology Service Ltd.
[0062] 4. Experimental groups: 8 mice for each group, with half males and half females.
[0063] rabies vaccine
[0064] rabies vaccine+1.25 μg CpG667
[0065] rabies vaccine+1.25 μg CpG705
[0066] rabies vaccine+1.25 μg CpG309
[0067] rabies vaccine+1.25 μg CpG205
[0068] rabies vaccine+5 μg CpG667
[0069] rabies vaccine+5 μg CpG705
[0070] rabies vaccine+5 μg CpG309
[0071] rabies vaccine+5 μg CpG205
[0072] rabies vaccine+20 μg CpG667
[0073] rabies vaccine+20 μg CpG705
[0074] rabies vaccine+20 μg CpG309
[0075] rabies...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com