Method for producing rabies vaccine by applying bioreactor and sheet carrier
A bioreactor, rabies vaccine technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of long production cycle, low virus harvest, unsuitable for large-scale production, etc., and achieve production costs Effect of low, high cell density, high viral titer and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
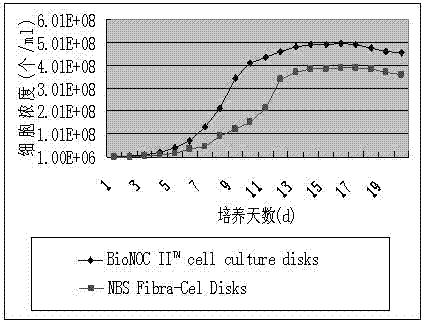
[0039] (1) Sterilize the Celligen 310 14L bioreactor with a fixed bed basket stirring system and assemble it completely, add cell culture medium, add BioNOC II? cell culture disks microcarrier 15g / L, calf serum content in the culture medium is 5 %, adjust the pH to 7.0, temperature at 33°C, and stirring speed at 20rpm overnight; inoculate Vero cells amplified by 3L spinner bottles at a seeding density of 0.5×10 6 cells / ml, adjust CO 2 The pressure is 5%, the pH is 7.2, the temperature is 35°C, the dissolved oxygen is 20%, the glucose concentration is 25uM, and the stirring speed is 20rpm;
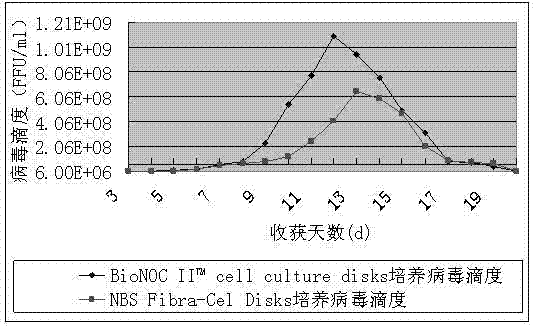
[0040] (2) When the cell density increases to 5×10 7 cells / ml was inoculated with aGV virus, the inoculation ratio was 1:100,
[0041] (3) Detect parameters such as glucose and lactic acid content through a biochemical analyzer, and adjust the reactor control parameters: CO 2 The pressure is 5%, the pH is 7.4, the temperature is 30°C, the dissolved oxygen is 20%, the glucose concentratio...
Embodiment 2
[0044] (1) Sterilize the Celligen 310 14L bioreactor with a fixed bed basket stirring system and assemble it completely, add cell culture medium, add BioNOC II? cell culture disks microcarrier 18g / L, calf serum content in the culture medium is 8 %, adjust the pH to 7.2, temperature 35°C, and stirring speed 35rpm overnight; inoculate Vero cells amplified by 3L spinner bottles, and the inoculation density is 1.3×10 6 cells / ml, adjust CO 2 The pressure is 8%, the pH is 7.3, the temperature is 36°C, the dissolved oxygen is 55%, the glucose concentration is 35uM, and the stirring speed is 60rpm;
[0045] (2) When the cell density increases to 5×10 7 cells / ml was inoculated with aGV virus, the inoculation ratio was 1:300,
[0046] (3) Detect parameters such as glucose and lactic acid content through a biochemical analyzer, and adjust the reactor control parameters: CO 2 The pressure is 8%, the pH is 7.6, the temperature is 33°C, the dissolved oxygen is 55%, the glucose concentrat...
Embodiment 3
[0049] (1) Sterilize the Celligen 310 14L bioreactor with a fixed bed basket stirring system and assemble it completely, add cell culture medium, add BioNOC II? cell culture disks microcarrier 20g / L, calf serum content in the culture medium is 10 %, adjust the pH to 7.4, temperature at 37°C, and stirring speed at 50rpm overnight; inoculate Vero cells amplified by 3L spinner bottles at a seeding density of 2×10 6 cells / ml, adjust CO 2 The pressure is 10%, the pH is 7.4, the temperature is 37°C, the dissolved oxygen is 90%, the glucose concentration is 40uM, and the stirring speed is 100rpm;
[0050] (2) When the cell density increases to 5×10 7 cells / ml was inoculated with aGV virus, the inoculation ratio was 1:500,
[0051] (3) Detect parameters such as glucose and lactic acid content through a biochemical analyzer, and adjust the reactor control parameters: CO 2 The pressure is 10%, the pH is 7.8, the temperature is 35°C, the dissolved oxygen is 90%, the glucose concentrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com