Modified nucleosides, nucleotides, and nucleic acids, and uses thereof
a technology of nucleosides and nucleic acids, applied in the field of modified nucleic acids, can solve the problems of difficult to obtain dna expression in cells, alterations and/or damage to the genomic dna of host cells, etc., and achieve the effect of reducing the innate immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Modified mRNA In Vitro Transcription
A. Materials and Methods
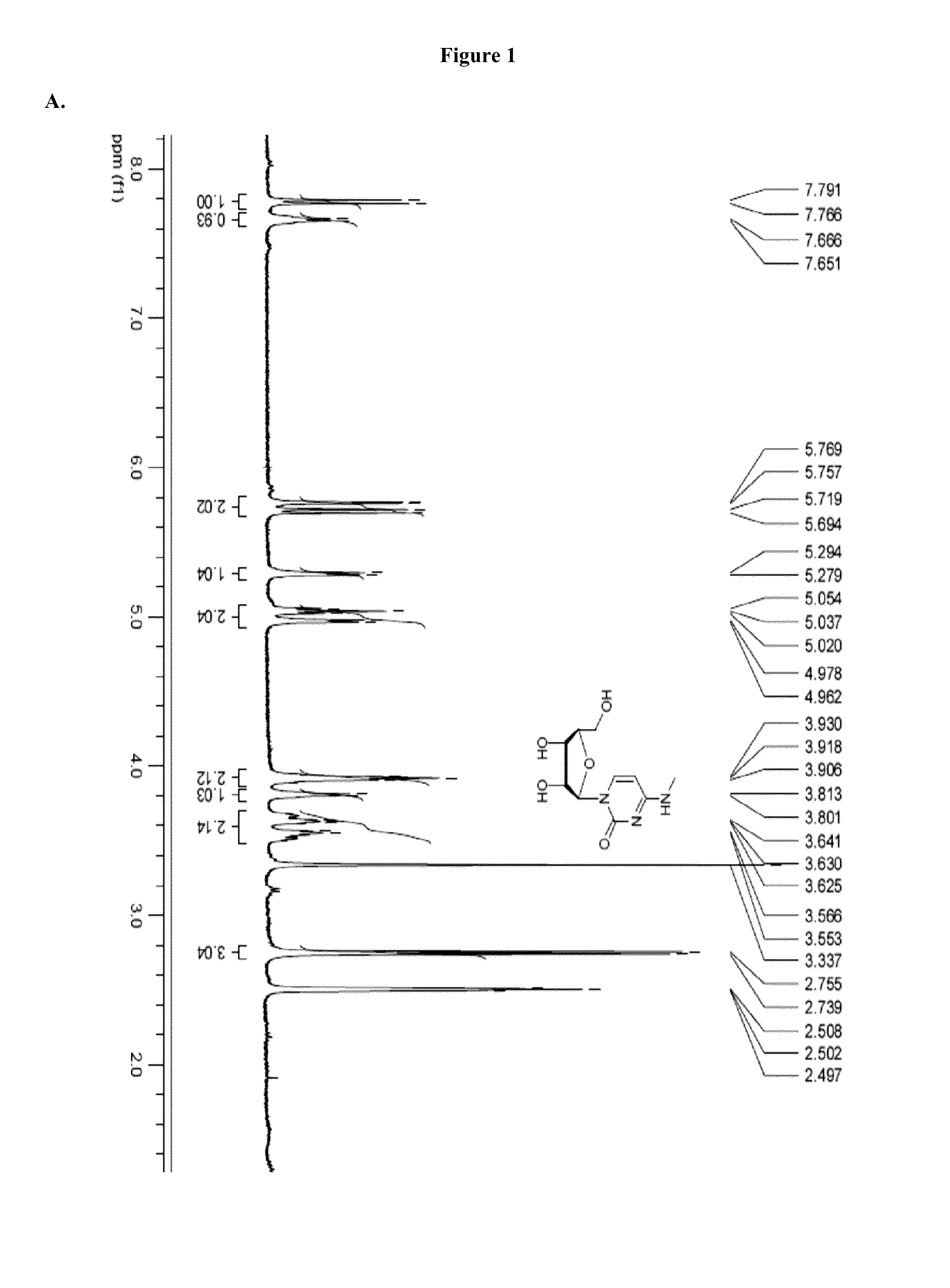
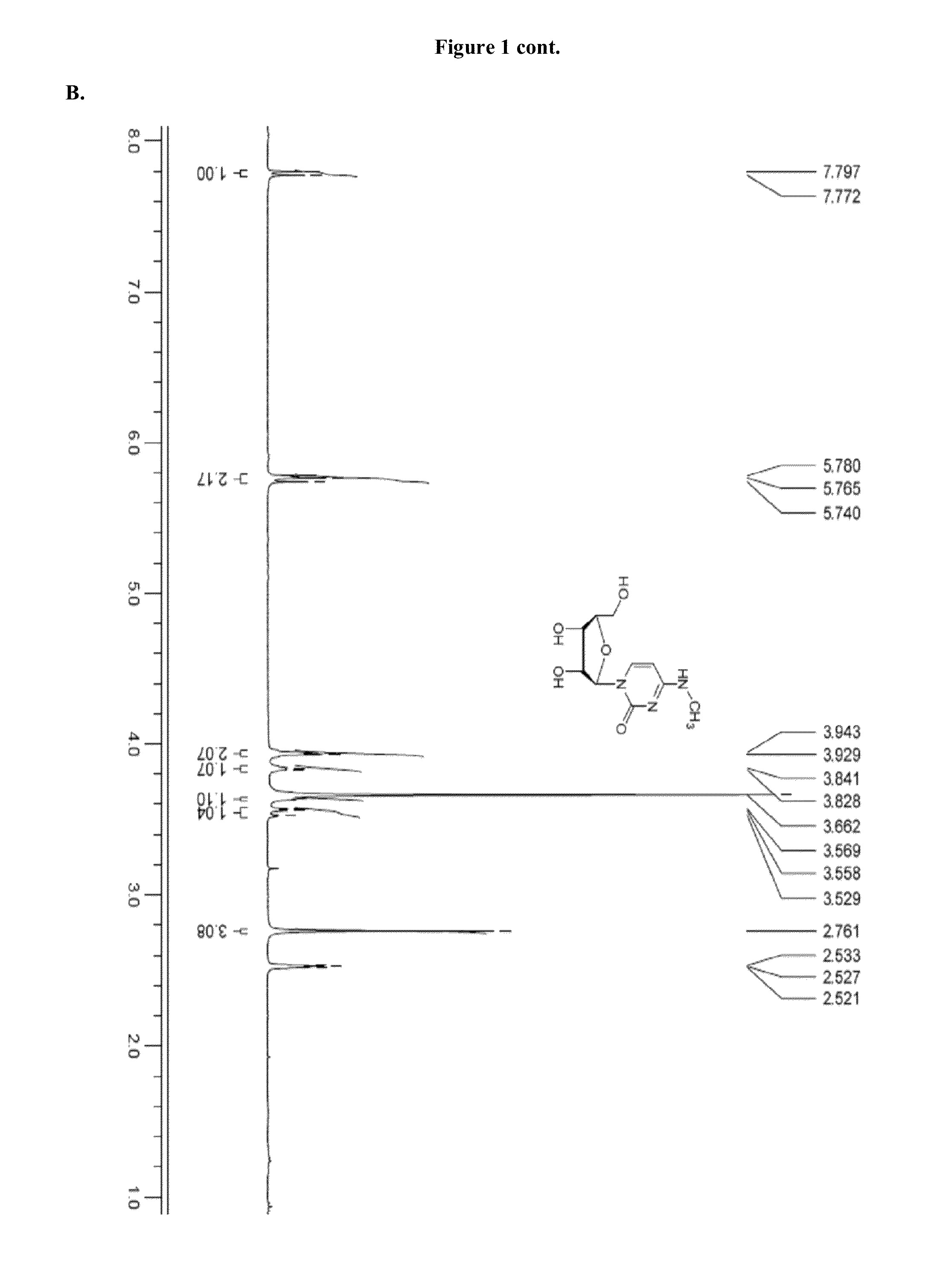
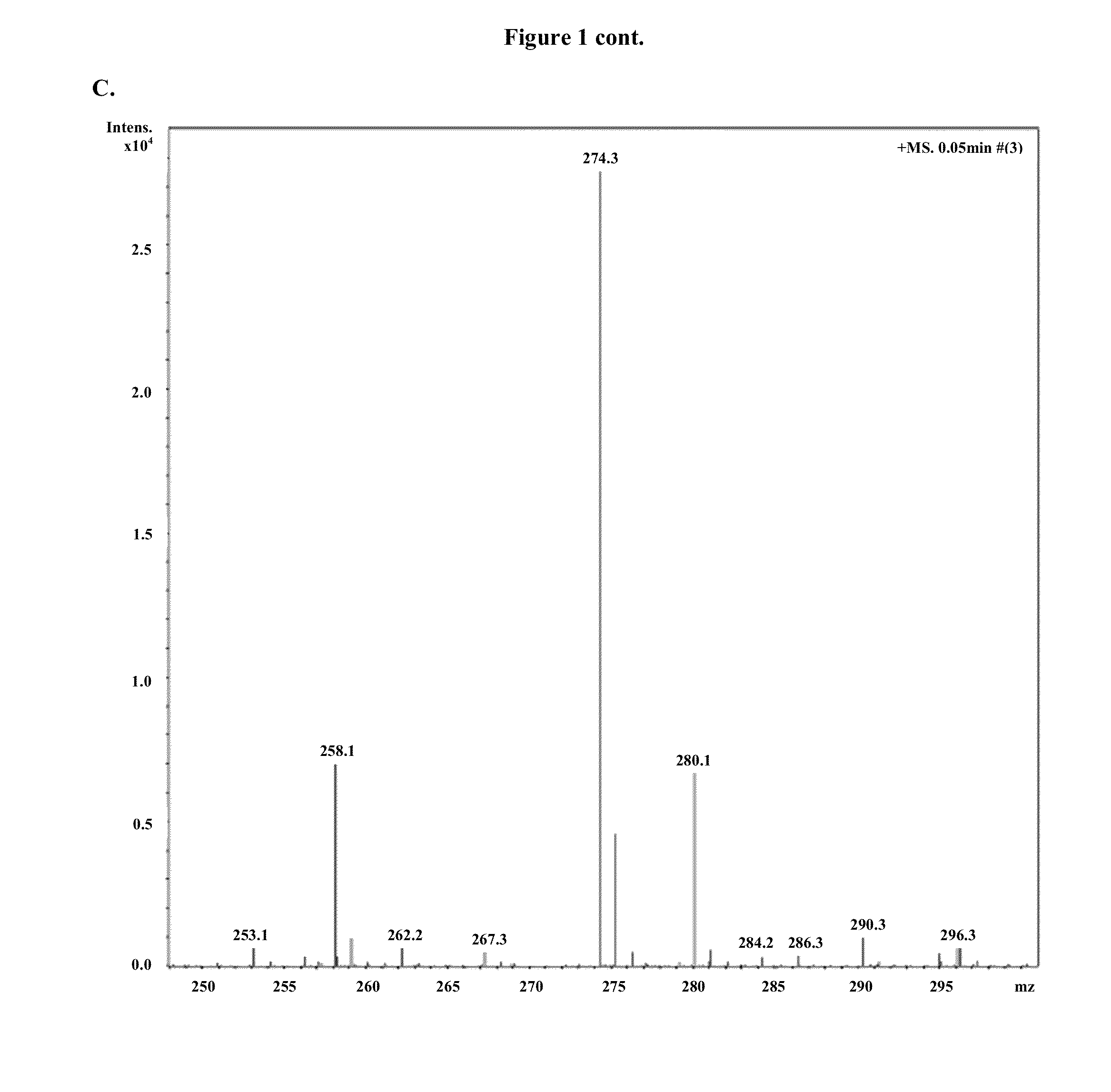
[0826]Modified mRNAs according to the invention are made using standard laboratory methods and materials for in vitro transcription with the exception that the nucleotide mix contains modified nucleotides. The open reading frame (ORF) of the gene of interest is flanked by a 5′ untranslated region (UTR) containing a strong Kozak translational initiation signal and an alpha-globin 3′ UTR terminating with an oligo(dT) sequence for templated addition of a polyA tail for mRNAs not incorporating adenosine analogs. Adenosine-containing mRNAs are synthesized without an oligo (dT) sequence to allow for post-transcription poly (A) polymerase poly-(A) tailing.
[0827]The modified mRNAs may be modified to reduce the cellular innate immune response. The modifications to reduce the cellular response may include pseudouridine (ψ) and 5-methyl-cytidine (5meC, 5 mc or m5C). (See, Kariko K et al. Immunity 23:165-75 (2005), Kariko K et al. Mol ...
example 2
Modified mRNA Transfection
A. Reverse Transfection
[0846]For experiments performed in a 24-well collagen-coated tissue culture plate, Keratinocytes are seeded at a cell density of 1×105. For experiments performed in a 96-well collagen-coated tissue culture plate, Keratinocytes are seeded at a cell density of 0.5×105. For each modified mRNA to be transfected, modified mRNA: RNAIMAX™ are prepared as described and mixed with the cells in the multi-well plate within 6 hours of cell seeding before cells had adhered to the tissue culture plate.
B. Forward Transfection
[0847]In a 24-well collagen-coated tissue culture plate, Keratinocytes are seeded at a cell density of 0.7×105. For experiments performed in a 96-well collagen-coated tissue culture plate, Keratinocytes are seeded at a cell density of 0.3×105. Keratinocytes are then grown to a confluency of >70% for over 24 hours. For each modified mRNA to be transfected, modified mRNA: RNAIMAX™ are prepared as described and transfected onto the...
example 3
Cellular Innate Immune Response to Modified Nucleic Acids: IFN-Beta ELISA and TNF-Alpha ELISA
[0851]An enzyme-linked immunosorbent assay (ELISA) for Human Tumor Necrosis Factor-α (TNF-α), Human Interferon-β (IFN-β) and Human Granulocyte-Colony Stimulating Factor (G-CSF) secreted from in vitro-transfected Human Keratinocyte cells is tested for the detection of a cellular innate immune response.
[0852]Keratinocytes are grown in EPILIFE® medium with Human Keratinocyte Growth Supplement in the absence of hydrocortisone from Invitrogen at a confluence of >70%. Keratinocytes are reverse transfected with 0 ng, 93.75 ng, 187.5 ng, 375 ng, 750 ng, 1500 ng or 3000 ng of the indicated chemically modified mRNA complexed with RNAIMAX™ from Invitrogen as described in triplicate. Secreted TNF-α in the culture medium is measured 24 hours post-transfection for each of the chemically modified mRNAs using an ELISA kit from Invitrogen according to the manufacturer protocols.
[0853]Secreted IFN-β is measur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com