Modified Vaccinia virus Ankara for the vaccination of neonates
a technology of vaccinia virus and ankara, which is applied in the field of modified vaccinia virus ankara for the vaccination of neonates, to achieve the effects of increasing the activation level of factors, increasing the number of dendritic cells, and facilitating the initiation of vaccinia virus replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(I) MVA-BN and DISC-HSV induces DC of the CD11c+and CD8+phenotype in newborn animals
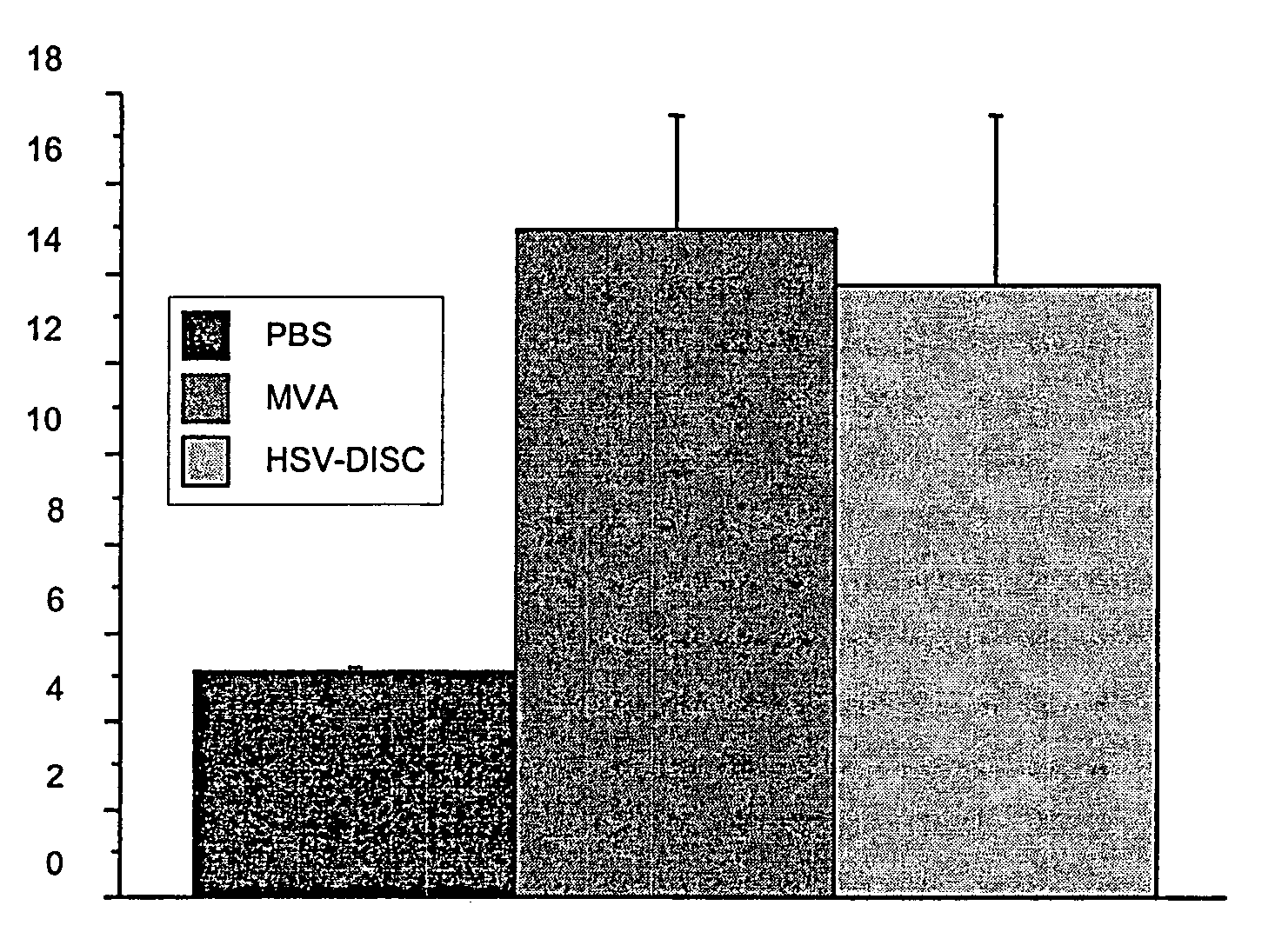
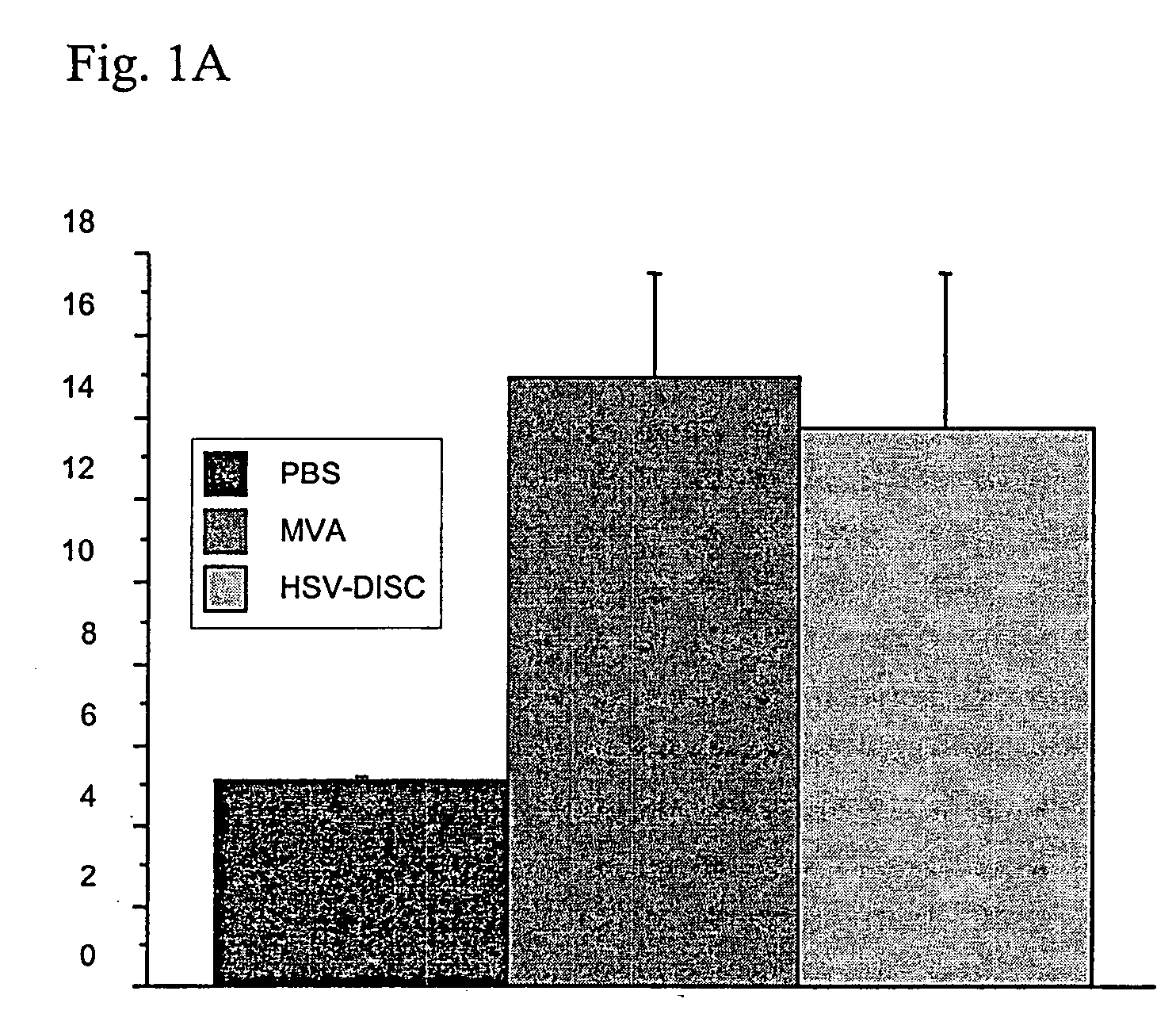
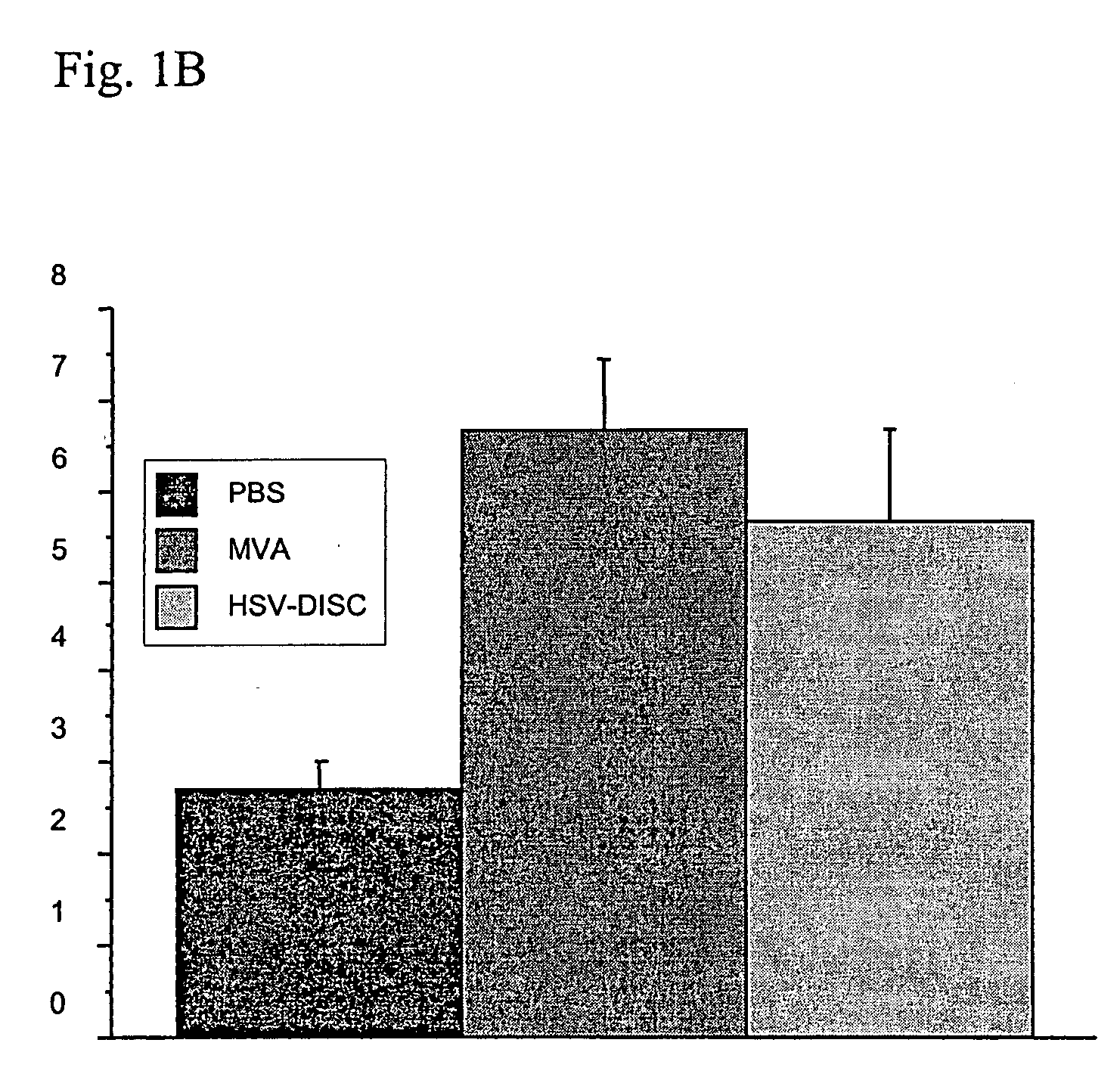
[0142] First set of experiments: Newborn mice are naturally immunodeficient because the IFN system is not mature. The number and activation state of DC, the best producers of IFN know today has not been analyzed. DC can be induced in vitro as well as in vivo by a variety of stimuli. In these studies it was tested whether a controlled MVA-BN replication could induce DC and analyzed their phenotype. Groups of mice were injected with 106 plaque forming units (p.f.u.) of MVA-BN or saline only within 1-2 days after birth and in some cases 5 days after birth. Blood and spleen cells from individual mice of both groups were analyzed by FACS and the data compared.
[0143] Data from 7 to 8 individual mice indicated that treatment with MVA-BN increased CD11c+cells 2-3 fold accompanied with increased expression of MHC II and increased presence of T cells of the CD4 or CD8 type Interestingly, CD19 / 54, a marker fo...
example 2
[0154] (i) MVA-BN treated neonatal mice survive a challenge with 100 to 500 LD50 of HSV-1
[0155] Groups of mice were treated with the standard dose of MVA-BN one or 2 days after birth and challenged at 7-8 days of age with 100 to 500 LD50 of Herpes simplex virus 1 (HSV-1) (FIG. 4). MVA BN treated mice survived the challenge with HSV 1, whereas all the control mice died within 5-6 days after inoculating the challenge virus.
[0156] To further support these observations, 9 challenge experiments were performed with 40 MVA BN treated and 45 control mice. More than 80 % of the virus treated mice survived the challenge, whereas all the control mice died (FIG. 5).
[0157] In a separate set of experiments the mice were treated at birth with MVA-BN (2.5×106 TCID50 mouse). At day 8 a challenge with either 103 (1 LD50) or 105 (100 LD50) PFU of HSV-1 was performed. Following MVA-BN vaccination 65% of the mice survived a viral dose that killed 100% of the control mice (100 LD50) and 90% survived a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com