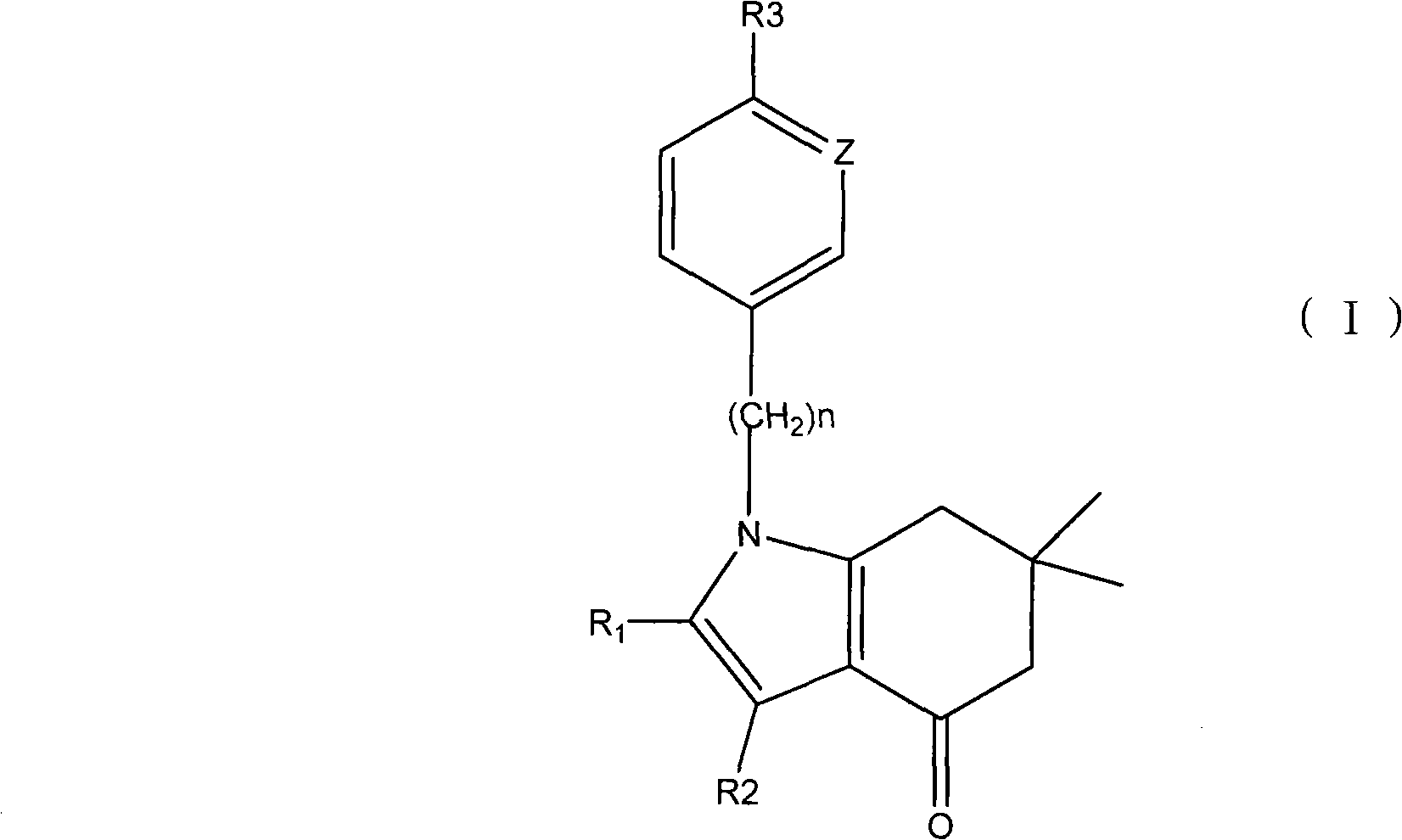
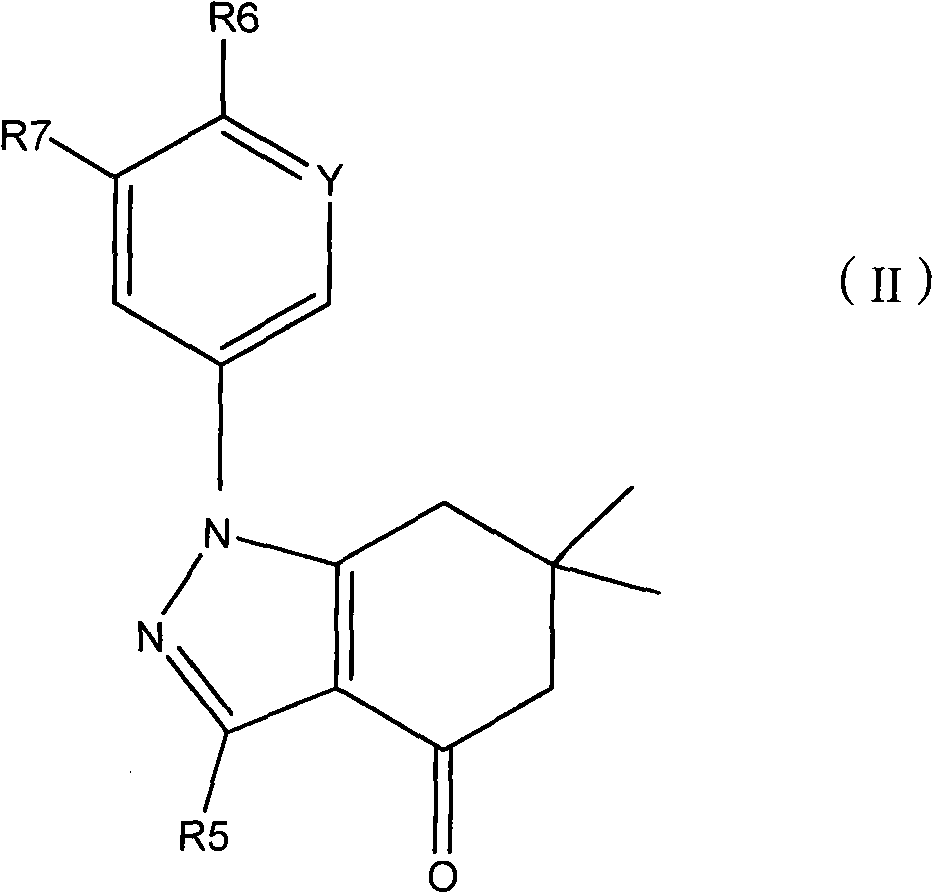
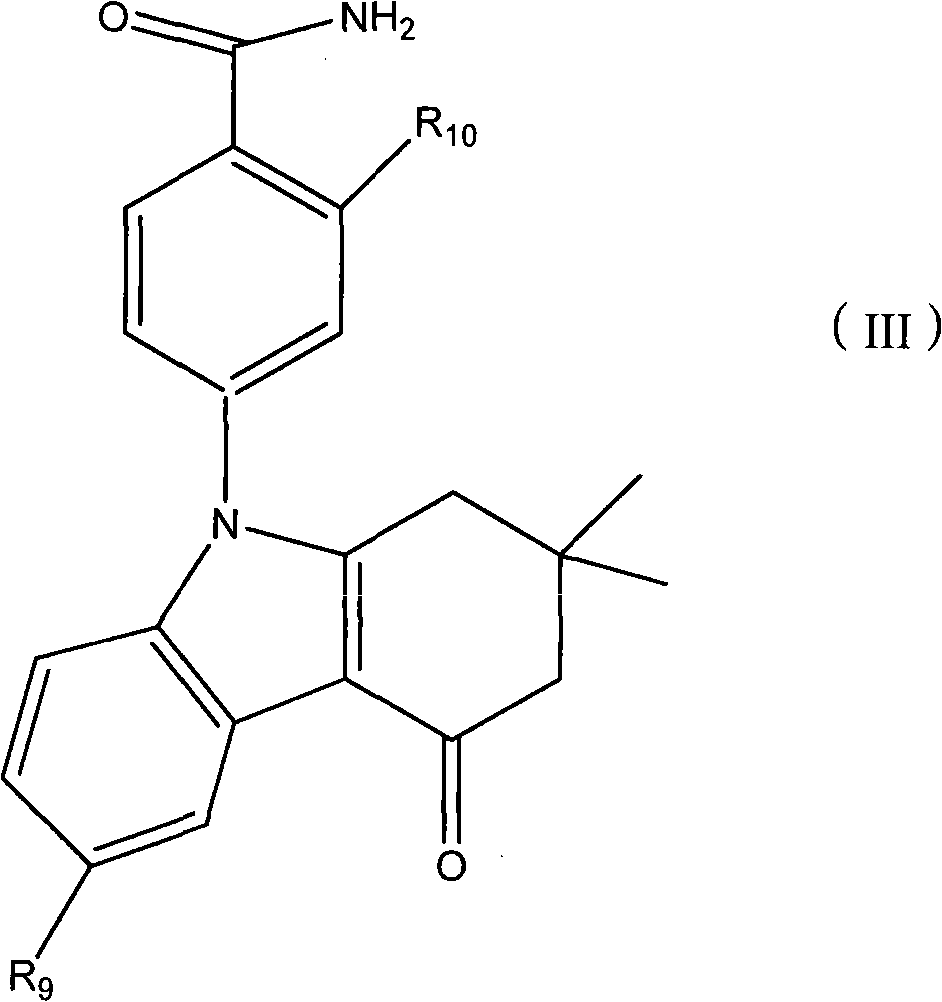
Applications of tetrahydroindolone/tetrahydroindazolone/tetrahydrocarbazole derivatives and salts thereof in preparation of antiviral medicine
An antiviral drug, the technology of tetrahydroindazolone, which is applied in the field of derivatives of tetrahydroindolinone/tetrahydroindazolone/tetrahydrocarbazole, can solve the problem of increasing the dose of spectral antiviral drugs, poor specificity, Poor efficacy and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, anti-herpes simplex virus type 1 (HSV1) experiment
[0045] (1) Instruments and reagents: imported 96-well culture plate, inverted microscope, CO 2 Incubator, micro-sampler; culture medium is DMEM (containing 250 U / ml of green chain double antibody), adjust pH to 7.0-7.2 with sodium bicarbonate before sterilization and filtration; calf serum is inactivated at 56°C for 30 minutes Complement, sterilized and aliquoted; digestive fluid 5% trypsin + 1% EDTA + 0.01mol / L (pH7.2) PBS, sterilized and aliquoted, stored at low temperature; cell growth medium: DMEM culture medium containing 10% FBS ; Cell maintenance fluid: DMEM culture fluid containing 1% FBS; Positive drug: acyclovir ACV; Test drug: tetrahydroindolinone / tetrahydroindazolone / tetrahydrocarbazole derivatives as described in Table 1 (Structural formulas I-01 to I-55).
[0046] (2) Virus and cell strain: herpes simplex virus type I virus strain HSV-1 (ATCC, VR733, F strain), through Vero cell subcultur...
Embodiment 2
[0072] Embodiment 2, anti-herpes simplex virus type II (HSV2)
[0073] (1) Instruments and reagents: refer to Example 1.
[0074] (2) Virus and cell strain: herpes simplex virus type II virus strain HSV-2 (provided by Wuhan Typical Species Preservation Center), and the titration of Vero cells is about 10 5 TCID 50 ; Cell line: African green monkey kidney cells (Vero), derived from American ATCC, CCL81 strain.
[0075] (3) Drug treatment: refer to Example 1.
[0076] (4) Measuring method: refer to Example 1.
[0077] (5) Cytotoxicity test of drugs: refer to Example 1.
[0078] (6) Antiviral activity test of medicine: refer to Example 1.
[0079] (7) Experimental results
[0080] 1) Cytotoxic MTT assay results:
[0081] The compounds of tetrahydroindolinone derivatives and tetrahydroindolinone derivatives all showed lower cytotoxicity to Vero cells, and their TC 50 See Table 2.
[0082] The maximum non-toxic concentration of each sample was selected as the maximum drug ...
Embodiment 3
[0088] Embodiment 3, anti-Coxsackie virus experiment
[0089] (1) Instruments and reagents: imported 96-well culture plate, inverted microscope, CO 2 Incubator, micro-sampler; culture medium is DMEM (containing 250 U / ml of green chain double antibody), adjust pH to 7.0-7.2 with sodium bicarbonate before sterilization and filtration; calf serum is inactivated at 56°C for 30 minutes Complement, sterilized and aliquoted; digestive fluid 5% trypsin + 1% EDTA + 0.01mol / L (pH7.2) PBS, sterilized and aliquoted, stored at low temperature; cell growth medium: DMEM culture medium containing 10% FCS Cell maintenance fluid: DMEM culture fluid containing 1%FCS; Positive drug: ribavirin; Test drug: the compound described in Table 1 of Tetrahydroindole derivatives and Tetrahydroindazole derivatives (structural formula sees I- in Table 1 01 to I-55).
[0090] (2) Cells and virus strains Cells: cervical cancer cells (Hela), derived from ATCC in the United States, CCL81 strain virus: CVB3 (pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com