Quadrivalent subunit influenza vaccine and preparation method thereof
An influenza vaccine and subunit technology, applied in the field of tetravalent subunit influenza vaccine, can solve problems such as limited influenza prevention, and achieve the effects of low adverse reactions, low formaldehyde and lysing agent residues, and a wide range of defense protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
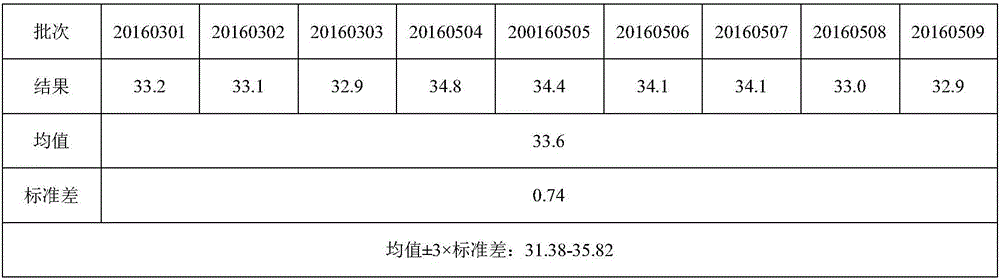
[0059] A quadrivalent subunit influenza vaccine, wherein each dose of the quadrivalent influenza subunit vaccine contains A1 (H1N1) type, A3 (two lineages of (H1N3) type and B (B) type), each valent antigen content It is 30~36μg / mL.
[0060] 1. Virus Inoculation and Culture
[0061] After diluting the batch virus species of the working seeds to a final concentration within the range of 2.0lgEID50 / mL to 5.0lgEID50 / mL (various types of influenza strains should be inoculated according to the same virus titer), inoculate chickens at a dose of 0.2mL / embryo. Embryo allantoic cavity, cultured at 33-35°C for 48-72 hours, unused working seeds to batch virus species, shall not be thawed for further use.
[0062] 2. Virus Harvesting
[0063] Screen live chicken embryos, place them in cold embryos at 2-8°C for 12-24 hours, harvest the allantoic fluid and take samples for testing: microbial limit, hemagglutination titer.
[0064] 3. Centrifuge to clarify and combine
[0065] The allant...
experiment example 1 4
[0077] Experimental Example 1: Research on semi-finished product technology of quadrivalent influenza virus subunit vaccine
[0078] 1. Materials
[0079] Monovalent stock solution of four types of influenza virus
[0080] 2. Method
[0081] According to the hemagglutinin content of each monovalent stock solution, the semi-finished products of various types of influenza viruses were prepared according to the same hemagglutinin content (the amount of hemagglutinin preparation can be within the range of 30-36 μg / mL, and each type of influenza virus strain should be prepared according to the same hemagglutinin content every year). Lectin content for preparation), that is, semi-finished products. According to the volume of the required monovalent stock solution diluent as V and the monovalent stock solution hemagglutinin content C0, calculate the volume V0=V / (C0 / hemagglutinin preparation amount) of the required monovalent stock solution, 0.01mol / L phosphate buffer saline The vo...
Embodiment 2
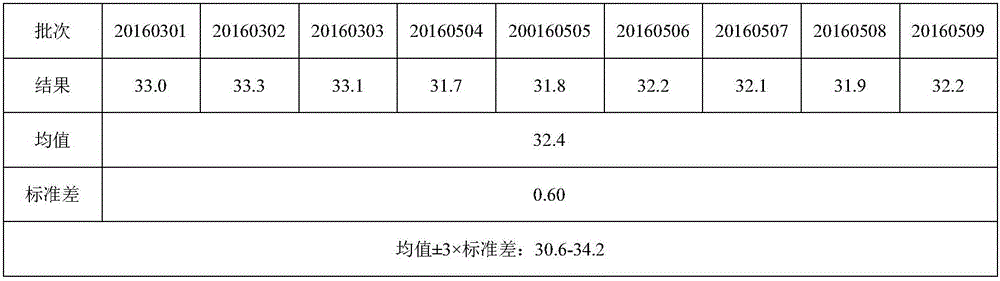
[0153] On the basis of Example 1, the density gradient centrifugation technique was replaced by the hydrophobic chromatography technique.
[0154] The specific method is: prepare the lysing agent Triton N101 with a concentration not higher than 1.5%, place it at 15-25° C., and lyse the virus for 0.5-2 hours. Afterwards, the different serotype samples were separated and purified using a hydrophobic chromatography column Butyl-S-Sepharose 6ff (C4). The chromatography medium is butylff, Octylff or PhenylF, and the loading buffer is 50mmol / L PBS+1.5mol / L (NH 4 ) 2 SO 4 (pH7.0), containing 100% ammonium sulfate, and set the ammonium sulfate content in 50mmol / L PBS as 0. After loading an appropriate amount of sample, the flow rate to ensure the column pressure will be eluted from the set ammonium sulfate content of 100% to 0 at a constant speed. , monitored at a wavelength of 280nm, and collected protein peaks.
[0155] The samples after hydrophobic chromatography were analyzed,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com