Quadrivalent influenza virus subunit vaccine and preparation method thereof
A subunit vaccine and influenza virus technology, applied in the field of quadrivalent influenza virus subunit vaccine and preparation thereof, can solve problems such as intractable induration itching, and achieve the effects of easy process, reduced redness and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
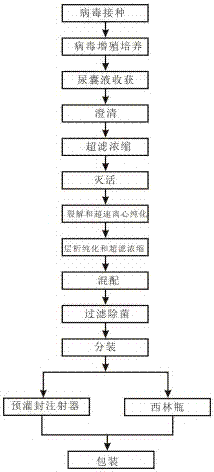
[0030] A 1 (H1N1) type influenza virus subunit vaccine monovalent stock solution production method, including the following steps in sequence:
[0031] (1). Virus inoculation: inoculate the working seeds of A1 (H1N1) influenza virus in the allantoic cavity of 10-day-old healthy chicken embryos;
[0032] (2). Virus propagation culture: transfer the inoculated chicken embryos to a 34°C incubator for 48 hours to propagate influenza virus;
[0033] (3). Harvesting of allantoic fluid: screen live chicken embryos, place the embryos at 2-8°C for 16 hours, and then harvest the allantoic fluid;
[0034] (4). Clarification: remove most impurities such as chicken embryo red blood cells by centrifugation.
[0035] (5). Inactivation: add formaldehyde with a final concentration not higher than 200mg / ml to the monovalent virus harvest solution, and inactivate at 2-8°C for 80 hours;
[0036] (6). Ultrafiltration concentration: the influenza virus allantoic harvest liquid after fully inactiv...
Embodiment 2
[0040] A 3 (H3N2) type influenza virus subunit vaccine monovalent stock solution production method, including the following steps in sequence:
[0041] (1). Virus inoculation: Inoculate the working seeds of A3 (H3N2) influenza virus into the allantoic cavity of 10-day-old healthy chicken embryos;
[0042] (2). Virus propagation culture: transfer the inoculated chicken embryos to a 34°C incubator for 48 hours to propagate influenza virus;
[0043] (3). Harvesting of allantoic fluid: screen live chicken embryos, place the embryos at 2-8°C for 16 hours, and then harvest the allantoic fluid;
[0044] (4). Clarification: remove most impurities such as chicken embryo red blood cells by centrifugation.
[0045] (5). Inactivation: add formaldehyde with a final concentration not higher than 200mg / ml to the monovalent virus harvest solution, and inactivate at 2~8°C for 100 hours;
[0046] (6).Ultrafiltration and concentration: the fully inactivated influenza virus allantoic harvest liqu...
Embodiment 3
[0050] The method for producing monovalent stock solution of type B (B) influenza virus subunit vaccine comprises the following steps in sequence:
[0051] (1). Virus inoculation: inoculate the working seeds of type B (B) influenza virus in the allantoic cavity of 10-day-old healthy chicken embryos;
[0052] (2). Virus propagation culture: transfer the inoculated chicken embryos to a 34°C incubator for 72 hours to propagate influenza virus;
[0053] (3). Harvesting of allantoic fluid: screen live chicken embryos, place the embryos at 2-8°C for 16 hours, and then harvest the allantoic fluid;
[0054] (4). Clarification: remove most impurities such as chicken embryo red blood cells by centrifugation.
[0055] (5). Inactivation: add formaldehyde with a final concentration of not higher than 200 mg / ml to the monovalent virus harvest liquid or ultrafiltration concentrated liquid, and inactivate at 2-8°C for 120 hours;
[0056] (6). Ultrafiltration concentration: the influenza vir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com