Bivalent egg yolk antibody against DVH (duck virus hepatitis) as well as preparation method and application of bivalent egg yolk antibody
A duck viral hepatitis, egg yolk antibody technology, applied in the direction of virus/phage, antiviral immunoglobulin, botanical equipment and methods, etc., can solve the problems of lack of effective control measures and lack of cross protection of DVH, and achieve prevention and control. Duck viral hepatitis, significant social benefit, stable use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the duck viral hepatitis bivalent egg yolk antibody adopts the following steps: breeding of duck hepatitis virus DHAV-1 and DHAV-3 seed virus, preparation of vaccine for immunization, immunization of laying hens, and extraction of egg yolk antibody. The selected DHAV-1 strain in the embodiment of the present invention is DRL-62 strain, and DHAV-3 strain is SD1 strain, and its specific method is:
[0043] 1. Source of virus species Duck hepatitis virus DRL-62 strain (preserved by ATCC, deposit number VR-1313); SD1 strain was isolated from Pulaike Bioengineering Co., Ltd. (CCTCC deposit, deposit number: CCTCC NO.V201225).
[0044] 2. Propagation of virus seeds Dilute duck hepatitis virus DRL-62 strain virus seeds with sterilized PBS solution at 1:100, inoculate 9-11-day-old SPF chicken embryos through the allantoic cavity, 0.1ml per embryo. Continue incubation at 37°C, discard dead embryos within 48 hours, harvest dead chicken embryos within 48-9...
Embodiment 1
[0062] Example 1 Molecular biological identification of DHV SD1 isolates
[0063] 1. Duck hepatitis virus SD1 strain virus is a newly isolated and identified virus, which has the following characteristics:
[0064] 1.1 The clinical symptoms caused by DHAV-3 and DHAV-1 are very similar. The main manifestations are: sudden onset of ducklings, obvious neurological symptoms, convulsions, and death soon. After death, ducklings are in opisthotonus posture. The pathological changes showed that the liver was enlarged, and there were a large number of bleeding points and spots on the liver surface, and the bleeding became more obvious with the prolongation of the death time.
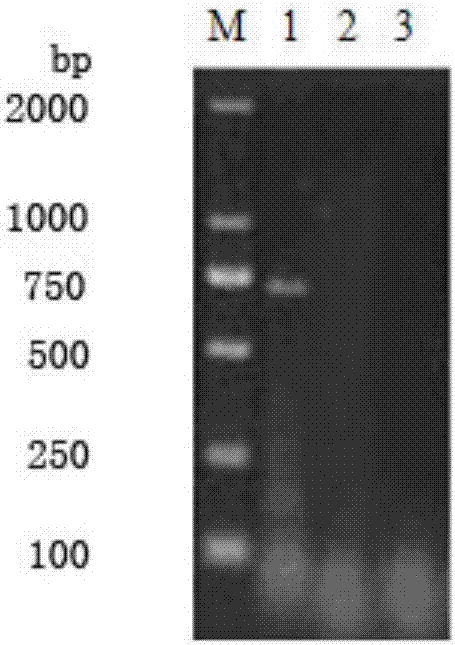
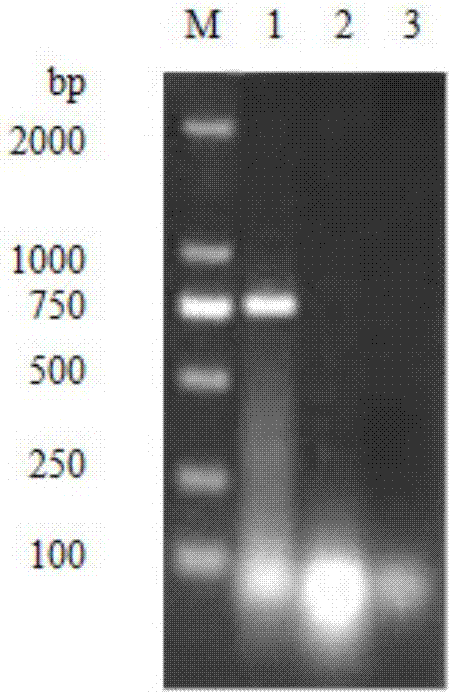
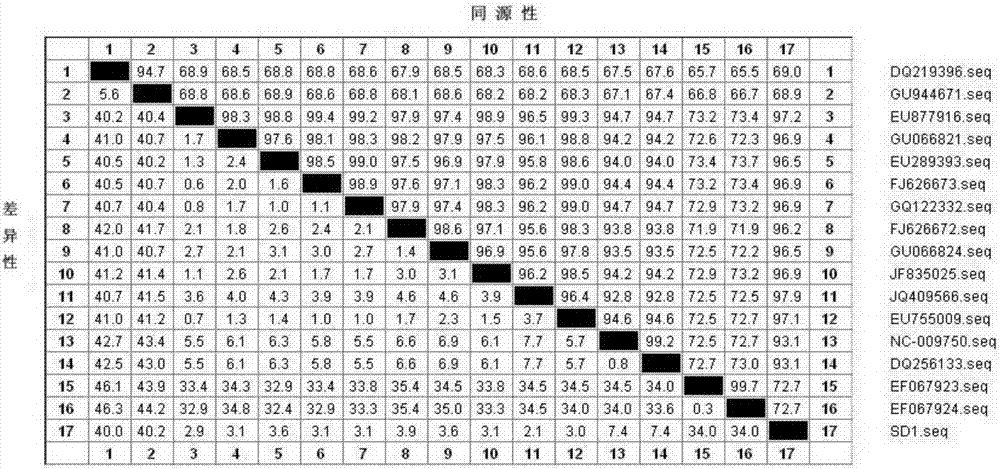
[0065] 1.2 According to the DHV genome sequence published in GenBank, several pairs of specific primers were designed, the VP1 gene was amplified by conventional RT-PCR method, and the sequence analysis of the VP1 gene of the isolate SD1 was carried out. The results showed that the VP1 gene of SD1 had the highes...
Embodiment 2
[0097] Embodiment 2 Serum cross-neutralization test
[0098] The serum was fixed and diluted, and the positive serum of the DHV DRL-62 strain and the SD1 isolate were serially diluted 2 times with normal saline. Take 1.0ml of the virus solution of DRL-62 strain and SD1 isolate containing 200ELD50 / 0.2ml and mix them with equal amounts of positive sera of different dilutions of each strain, and act at 37°C for 1h. At the same time, virus and saline controls were set. Each neutralization group and control group were inoculated with 5 duck embryos through the allantoic cavity, 0.2m1 / embryo, cultured at 37°C, observed for 7 days, and recorded the number of dead embryos in each group. The neutralization titer of the serum was defined as the highest serum dilution for 50% duck embryo protection.
[0099] The results of the neutralization test showed that the isolated strain could be completely neutralized by the positive serum of the isolated strain, but could not be neutralized an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com