Extractive and preparation containing same
一种提取物、制剂的技术,应用在痘苗病毒接种兔炎症皮肤提取物领域,能够解决没有启示、没有记载等问题,达到稳定确保、有效性和安全性恒定的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 (manufacture of this extract)
[0062] The skin of healthy mature rabbits was inoculated intradermally with vaccinia virus, and the pimple-prone skin was excised and harvested. After the collected skin is cleaned and disinfected with phenol solution, excess phenol solution is removed, crushed, mixed with phenol solution, left for 3-7 days, and heated at 35-40°C while stirring for 3-4 days. Then, the extract liquid obtained by solid-liquid separation was adjusted to pH 4.5-5.2 with hydrochloric acid, heat-treated at 90-100°C for 30 minutes, and then filtered to remove protein. Then adjust the filtrate to pH 9.0-9.5 with sodium hydroxide, heat treatment at 90-100° C. for 15 minutes, and then carry out solid-liquid separation.
[0063] The obtained protein-removing solution was adjusted to pH 4.0-4.3 with hydrochloric acid, and 2% of the mass of the protein-removing solution was added with activated carbon, stirred for 2 hours, and then solid-liquid separation w...
Embodiment 2
[0064] Embodiment 2 (assay method of N-acetylneuraminic acid content)
[0065] The N-acetylneuraminic acid content of this extract and this preparation was determined by high performance liquid chromatography mass spectrometry (LC-MS) as follows.
[0066] The extract (1.2 units / mL) prepared in Example 1 was diluted 10 times with water, and injected into LC-MS.
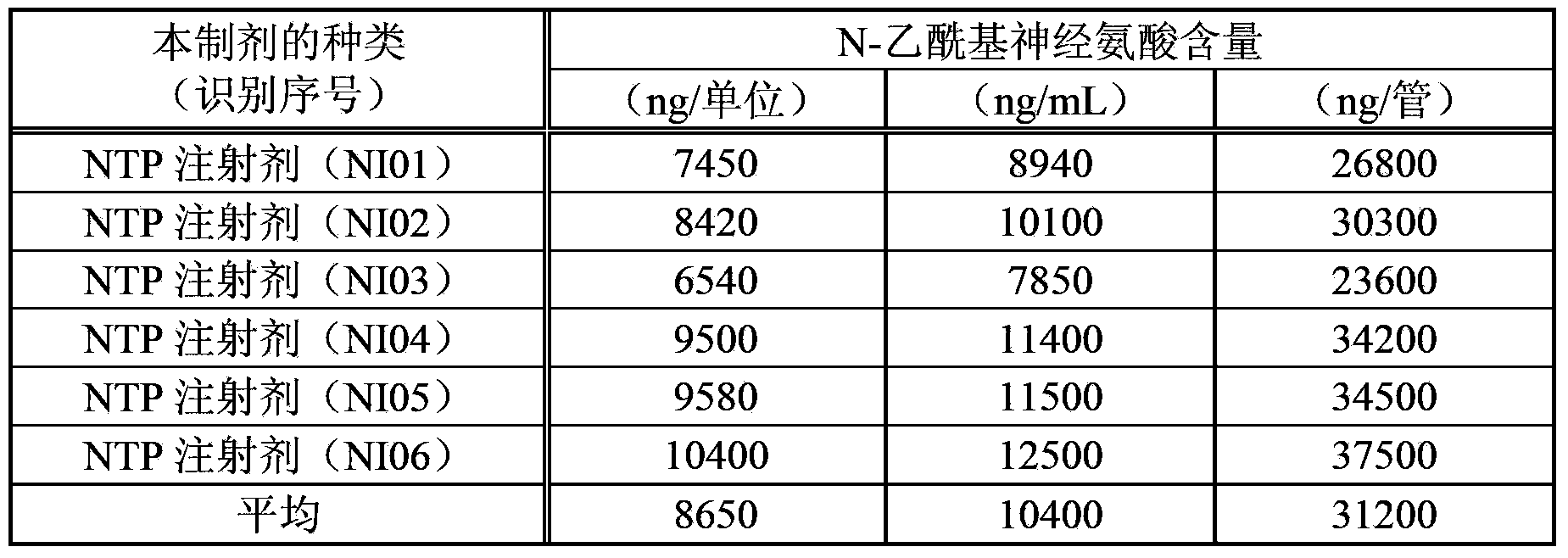
[0067] The formulation (NTP injection) produced using the extract produced in Example 1 was also diluted 10 times with water, and injected into LC-MS.
[0068] Using this preparation (NTP tablet) produced by using the extract produced in Example 1, wash 3 tablets with 3 mL of methanol / chloroform (1:1) 3 times, remove the film coating layer, dry, and add 12 mL Suspended in water (1 unit / mL), after centrifugation, the supernatant was diluted 10 times with water, and injected into LC-MS.
[0069] For N-acetylneuraminic acid, prepare a standard solution in aqueous solution to make a calibration curve.
[0070] For LC-MS...
Embodiment 3
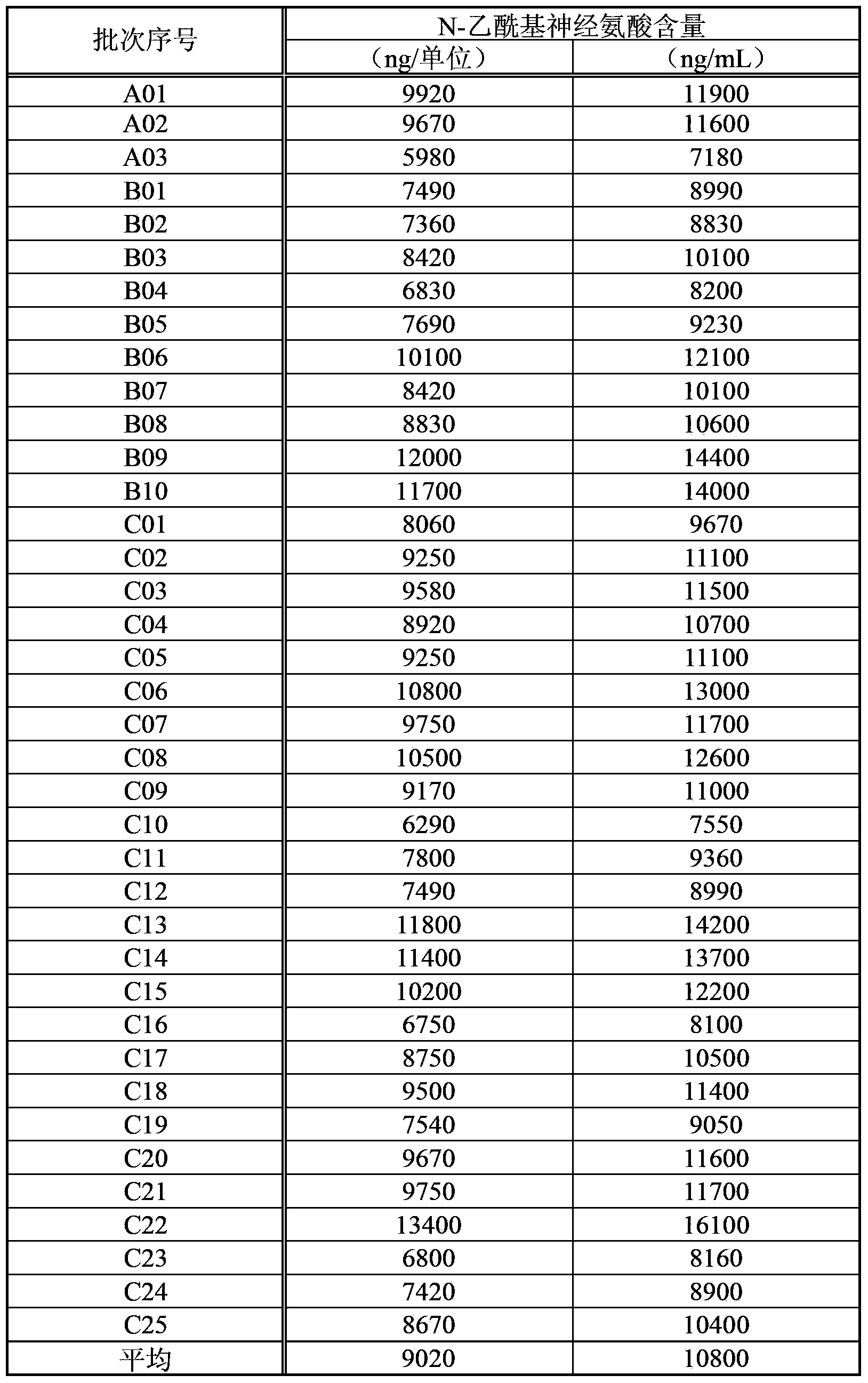
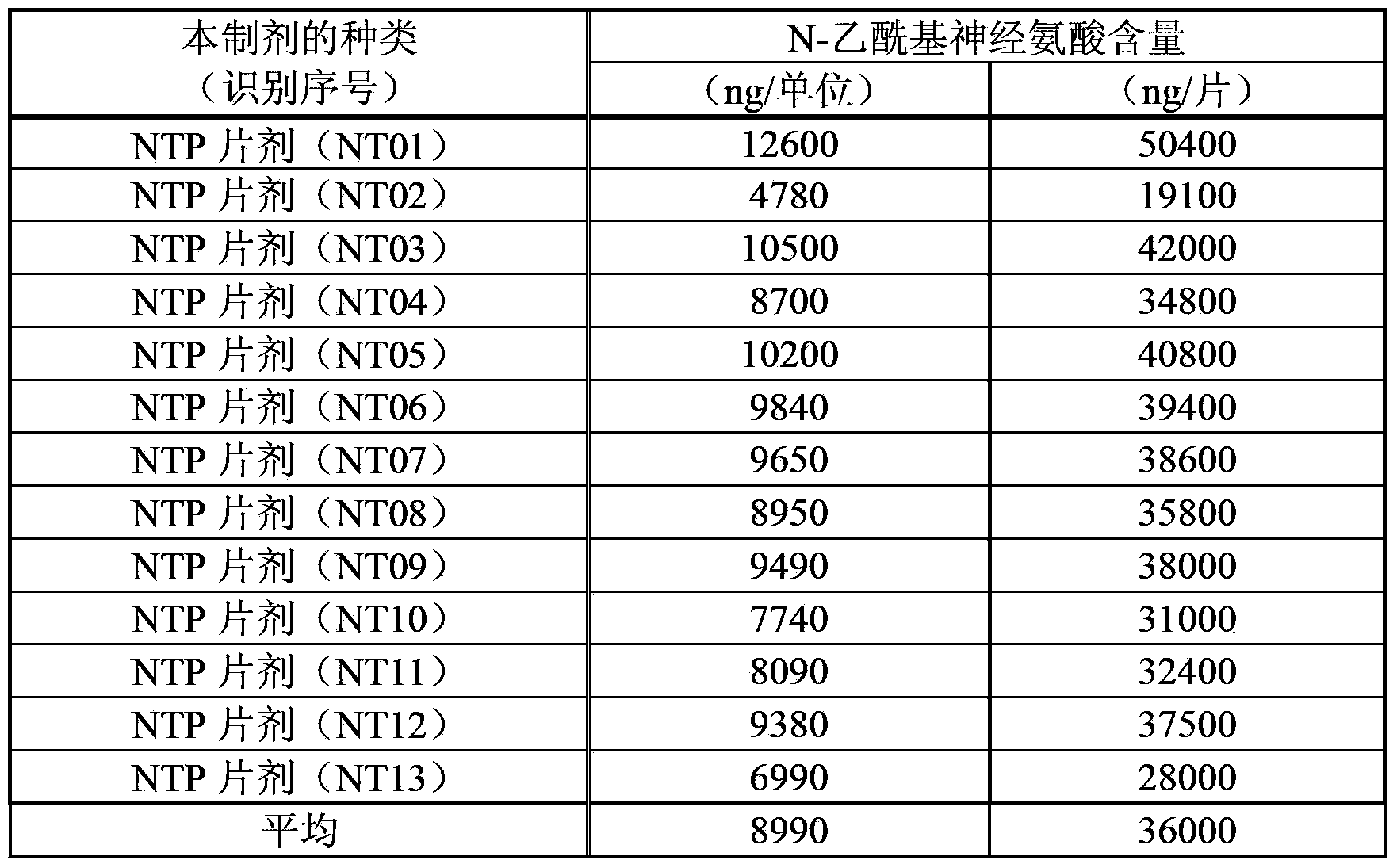
[0089] Example 3 (Measuring results of the N-acetylneuraminic acid content of the extract)
[0090] Table 2 shows the results of measuring the N-acetylneuraminic acid content in the present extract by the method described in Example 2 above. The active ingredient content in this extract is 1.2 units / mL. The content of N-acetylneuraminic acid in this extract is represented by both per 1 unit (" / unit") of this extract and per 1 mL (" / mL") of this extract. In addition, the symbols A to C in the lot number indicate the difference in the applicant's manufacturing location (facility). In addition, the measured values are unified to 3 significant figures (hereinafter, the same for all measured values).
[0091] [Table 2]
[0092]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com