Method for preparing swine fever live vaccines
A technology of swine fever live vaccine and silicone tube, which is applied in the direction of pharmaceutical formula, medical preparations containing active ingredients, recovery/purification, etc., to achieve the effects of improving sensitivity, ensuring titer, and uniform state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Cloning, purification and screening of cells for seedling production by limiting dilution method (porcine testis cells ST)
[0039] Porcine testicular cells ST were purchased from China Veterinary Drug Administration;
[0040] The classical swine fever virus vaccine strain is a rabbit-like attenuated strain of classical swine fever virus (strain C), which was purchased from China Veterinary Drug Control Institute;
[0041] The formula of the cell growth solution is 90% high-sugar DMEM solution by volume, 10% newborn bovine serum, and the pH value is adjusted to 7.4;
[0042] The cell maintenance solution is 96% high-sugar DMEM solution by volume, 4% newborn bovine serum, and the pH value is adjusted to 7.4;
[0043] A. Preparation of Monoclonal Cell Lines
[0044] a. Preparation of cell suspension
[0045] Porcine testicular cells were cultured in cell flasks, and when the cells were covered with a dense monolayer and the outline was clear, they were digest...
Embodiment 2
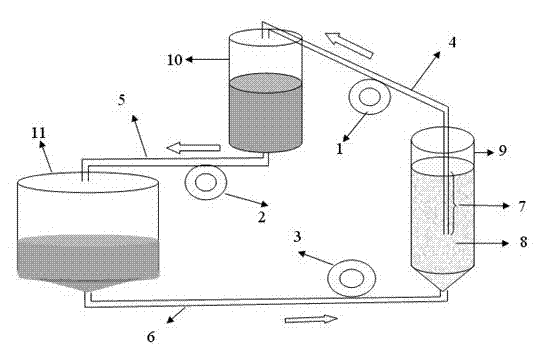
[0065] Embodiment 2 AP20C type torrent perfusion bioreactor prepares swine fever live vaccine
[0066] In the AP20C type torrent perfusion bioreactor used in this example, the perfusion bag 9 has a volume of 4.5 L, the mixer 10 has a volume of 8 L, the torrent tank 11 has a volume of 15 L, and the perfusion bag 9 contains 200 g of polyester fiber paper carrier 8; Theoretical effective culture volume is 18L.
[0067] The cells used for making seedlings are the same as the pig testicular cells (ST) preserved by the strains in Example 1;
[0068] The seed poison is the same as the attenuated strain (C strain) of swine fever rabbitization in Example 1, and a certain batch of seed poison titer is 5×10 5 RID / ml;
[0069] The cell growth solution is 92% high-sugar DMEM solution by volume, 8% newborn bovine serum, and the pH value is adjusted to 7.3;
[0070] The cell maintenance solution is 97% high-sugar DMEM solution by volume, 3% newborn bovine serum, and the pH value is adjust...
Embodiment 3
[0102] Example 3 AP200 Type Torrent Perfusion Bioreactor Preparation of Live Swine Fever Vaccine
[0103] The torrent perfusion bioreactors used in this example include AP20C type and AP200 type.
[0104] The AP20C type torrent perfusion bioreactor is the same as in Example 2.
[0105] The AP200 perfusion bag 9 has a volume of 45L, the mixer 10 has a volume of 80L, the surge tank 11 has a volume of 150L, and the perfusion bag 9 contains 2000g of polyester fiber paper carrier 8; the theoretical effective culture volume is 180L.
[0106] Seedlings are all the same as in Example 2 with cells and seed poisons.
[0107] A. to prepare
[0108] Two torrent perfusion bioreactors, AP20C and AP200, were selected to prepare for cell culture.
[0109] B, preparation work, concrete steps are as follows:
[0110] a. Cultivation of cells for seedling production
[0111] According to the method of Example 2, cultivate the cells for seedling production in the AP20C bioreactor for 96 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com