Methods of Preventing or Treating Respiratory Conditions
a respiratory condition and respiratory technology, applied in the field of respiratory conditions prevention or treatment protocols, can solve the problems of side effects, hypertension and drowsiness, and subject may develop arrhythmias or cardiogenic shocks, and achieve the effects of preventing recurrence, preventing the onset, and reducing or ameliorating the severity and/or duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6.1 Example 1
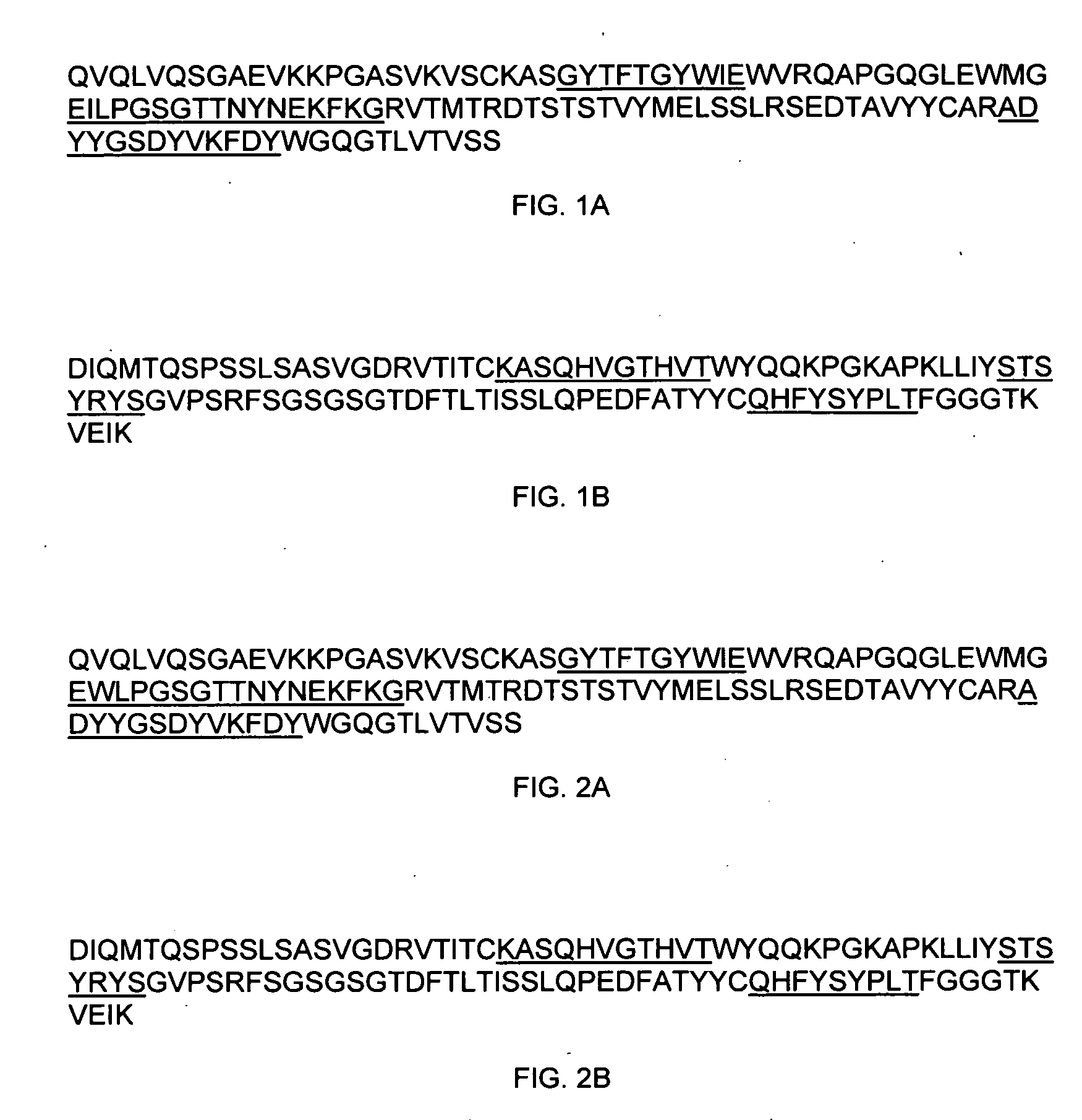
Preparation of IL-9 Antagonist MAb 7F3COM-2H2
[0589]Anti-human IL-9 antibody MAb 7F3com-2H2 was prepared using the pMI347 vector. The pMI347 vector coding for the expression of MAb 7F3com-2H2 consists of the following four independent genetic elements: a glutamine synthetase selectable marker expression cassette, the 7F3com-2H2 light chain cDNA expression cassette, the 7F3com-2H2 heavy chain mini-gene expression cassette, and a bacterial origin of replication and antibiotic resistance gene.
[0590]The first element, the glutamine synthetase selectable marker expression cassette consists of hamster glutamine synthetase (GS) cDNA under the control of simian virus 40 (SV40) early enhancer and promoter and SV40 early splicing and polyadenylation region for efficient mRNA cleavage and addition of a polyadneylate tail. This element is required for integration, amplification, and stable maintenance of the plasmid in the host cell genome.
[0591]Each of the 7F3com-2H2 heavy and ligh...
example 2
6.3 Example 2
Relationship Between IL-9 and Airway Hyperresponsiveness
[0654]This example demonstrates the relationship between IL-9 and airway hyperresponsiveness as well as the effectiveness of IL-9 antibodies in reducing airway hyperresponsiveness.
Materials and Methods
[0655]Experimental groups of four mice were acclimated to the plethysmography chamber and then exposed to aerosolized saline for two minutes. Penh values were obtained for five minutes after saline aerosol exposure to obtain a Penh baseline value for lung function. Subsequently, methylcholine chloride (“MCh”) (Sigma Aldrich, Sigma, Mo.) was introduced to the plethysmography chamber to induce bronchoconstriction in the mice. Penh values were monitored for five minutes after methylcholine chloride exposure. In some studies, 5 μg of recombinant murine IL-9 or BSA was administered once daily for three consecutive days. The mice were acclimated to the plesthymography chamber twenty-four (24) hours after the administration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com