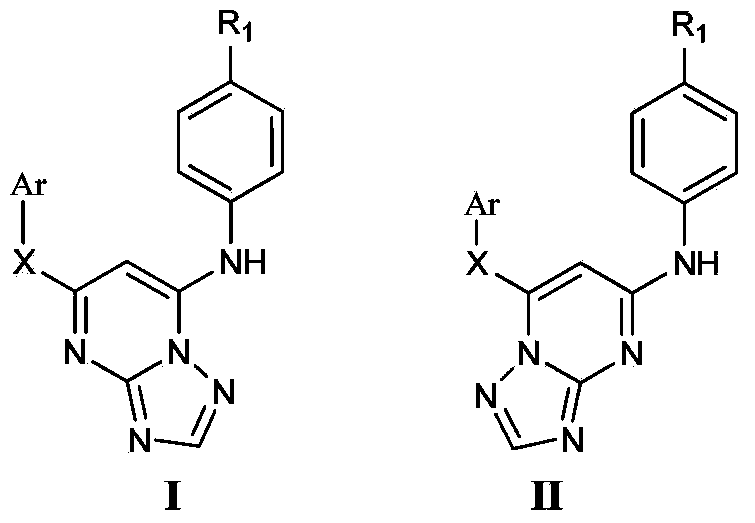
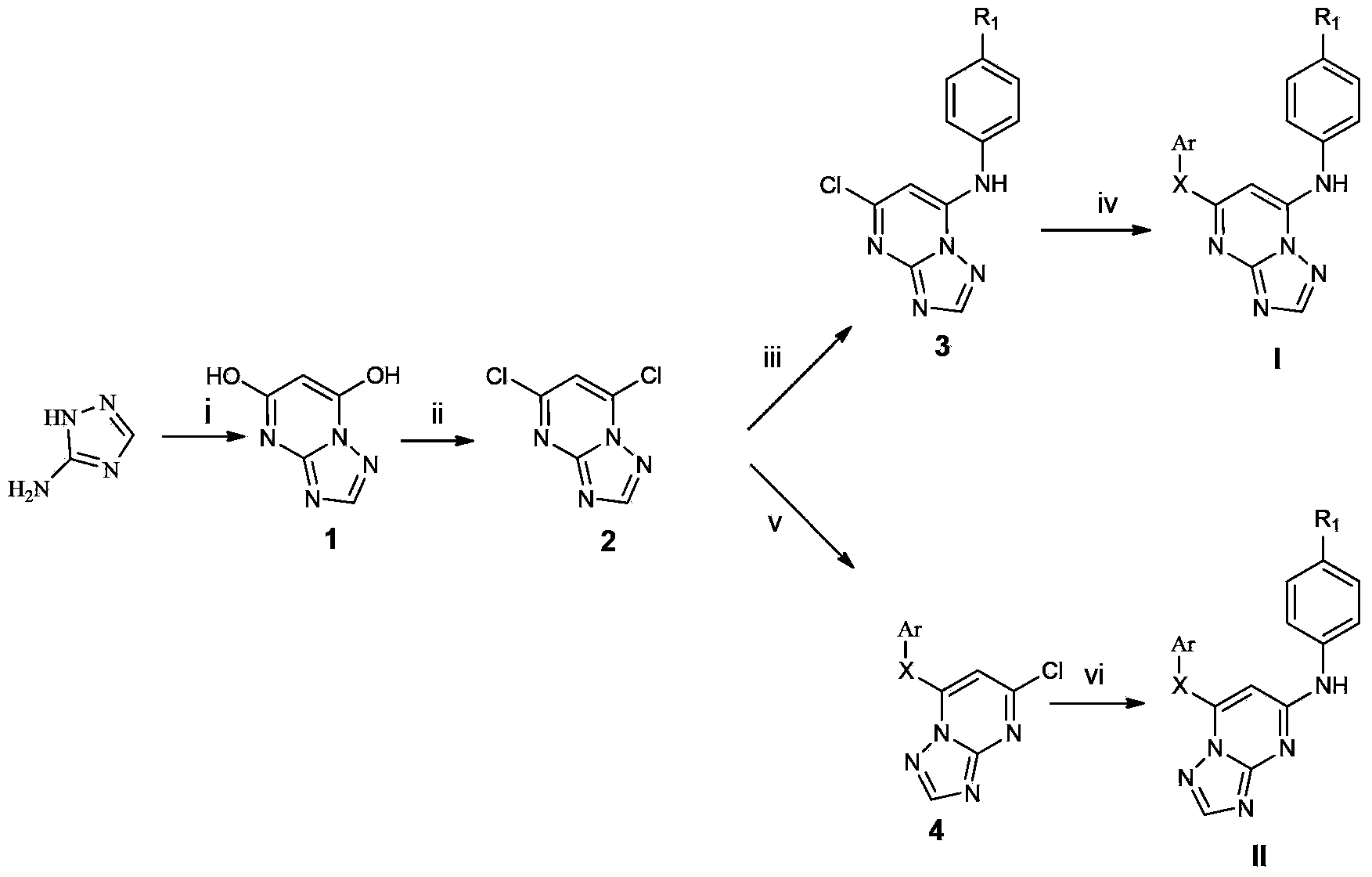
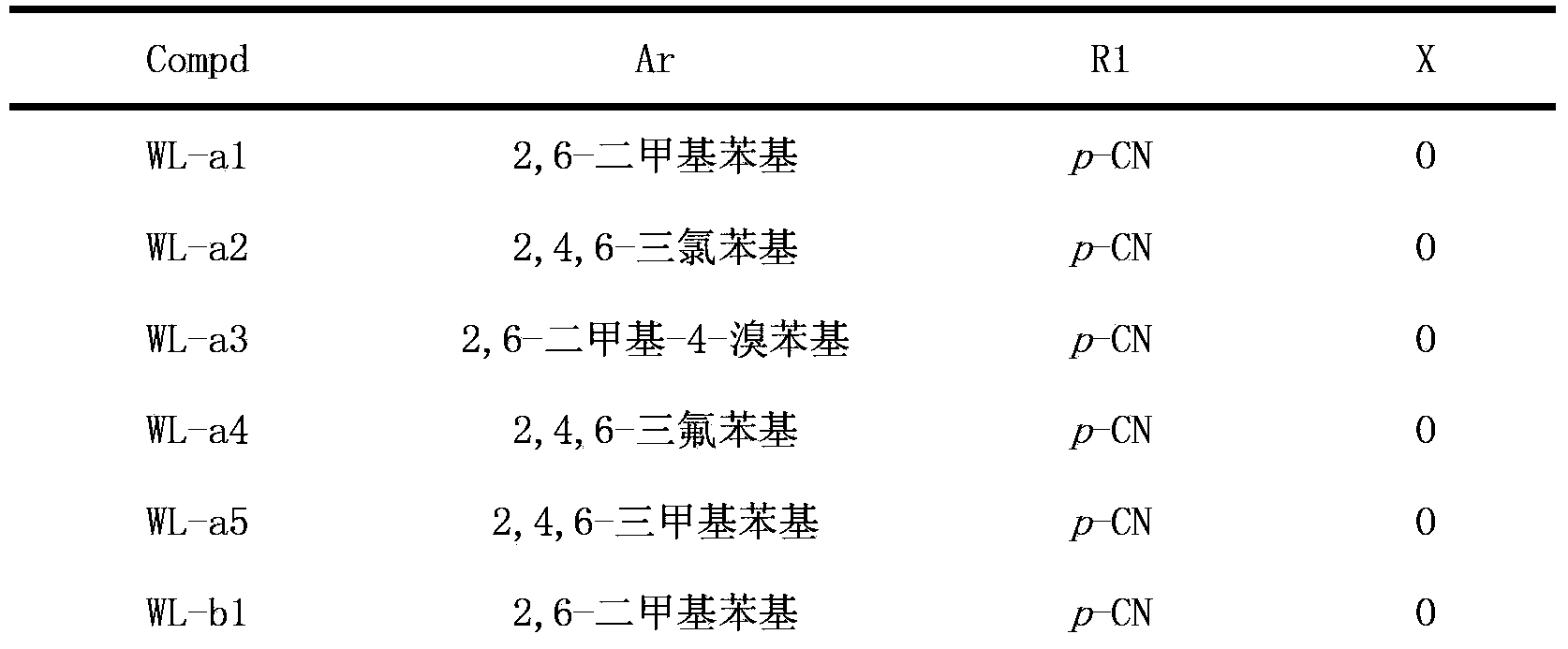
Triazolopyrimidine HIV-1 retrovirus inhibitor and its preparation method and application thereof
A reverse transcriptase inhibition, HIV-1 technology, applied in the field of medicine, can solve the problems of side effects, poor oral availability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1.4-((5-(2,4,6-trimethylphenoxy)-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)amino)benzonitrile (WL - Preparation of a1)
[0069] Add 5.0g (59.5mmol) of aminotriazole, 9.52g (59.5mmol) of diethyl malonate and 4.05g (59.5mmol) of NaOEt (prepared from Na and EtOH) into 50mL of anhydrous EtOH, stir, Reflux for 12h. The residual ethanol was distilled off, ice water was added, extracted three times with ethyl acetate, and diethyl malonate was removed. The aqueous layer was collected and acidified to pH=1 with concentrated HCl. Stand still in an ice bath to precipitate a large amount of white solid, filter, wash with water, and dry to obtain intermediate 1;
[0070] The prepared intermediate 16.08g (40mmol) was added to 57mL (600mmol) POCl 3 , stirred and refluxed at 95°C for 12h. Evaporate excess POCl 3 , add crushed ice after cooling, stir to remove residual POCl 3 . Join H 2 O and CH 2 Cl 2 Extraction was performed and the organic layer was collected. Take Na ...
Embodiment 2
[0074] Example 2.4-((5-(2,4,6-trichlorophenoxy)-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)amino)benzonitrile ( Preparation of WL-a2)
[0075] The preparation method is as in Example 1, and the difference is: the trichlorophenol of 2mmol and the K of 4mmol 2 CO 3 Add it into an appropriate amount of DMF, stir at room temperature for 5 min, then add 2 mmol of intermediate 3, and react at 150° C. for 8-12 h. The product is a white solid, the total yield is 7%, mp: 258-260°C.
[0076] ESIMS: m / z431.3(M+1), 433.4(M+3), 435.3(M+5), 437.3(M+7).
[0077] IR(KBr,cm -1 ):3303(NH),2231(CN).
[0078] 1 H NMR (DMSO-d 6 ,400MHz)δ:10.16(s,1H,NH),8.48(s,1H,triazole-H),8.13(s,2H,OPh-H),7.96(d,2H,J=8.8Hz,Ph-H ), 7.84 (d, 2H, J=8.8Hz, Ph-H), 5.91 (s, 1H, pyrimidine-H).
Embodiment 3
[0079] Example 3.4-((5-(4-bromo-2,6-dimethylphenoxy)-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)amino) Preparation of benzonitrile (WL-a3)
[0080] The preparation method is as in Example 1, the difference is: 2mmol of 4-bromo-2,6-dimethylphenol and 4mmol of K 2 CO 3 Add it into an appropriate amount of DMF, stir at room temperature for 5 min, then add 2 mmol of intermediate 3, and react at 150° C. for 8-12 h. The product is a white solid, 8% overall yield, mp: 285°C (dec).
[0081] ESIMS: m / z435.4(M+1),437.4(M+3).
[0082] IR(KBr,cm -1):3358(NH),2218(CN).
[0083] 1 H NMR (DMSO-d 6 ,400MHz)δ:10.13(s,1H,NH),8.44(s,1H,triazole-H),7.98(d,2H,J=8.8Hz,Ph-H),7.82(d,2H,J=8.8 Hz, Ph-H), 7.60 (s, 2H, OPh-H), 5.71 (s, 1H, pyrimidine-H), 2.18 (s, 6H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com