Method for detection of HIV-1 nucleoside inhibitor drug resistance mutation and kit
A technology for HIV-1 and drug-resistant mutations, applied in the direction of microbial-based methods, biochemical equipment and methods, and microbial measurement/testing, can solve problems such as time-consuming, undetectable, and lack of quality assurance of detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Embodiment 1, using the present invention to detect HIV-1 virus nucleoside reverse transcriptase inhibitor single-site drug-resistant mutation
[0123] In this embodiment, all HIV-1 virus nucleoside inhibitor drug-resistant mutation types involved in the present invention, as well as wild-type HIV-1 virus reverse transcriptase gene types that do not contain any mutations, are detected.
[0124] 1. Preparation of Oligonucleotide Primers and Probes
[0125] Table 1 and Table 2 show the sequences of primers and probes used in the detection of HIV-1 viral nucleoside reverse transcriptase inhibitor resistance mutations according to the present invention, all of which were synthesized by Shanghai Yingjun Biotechnology Co., Ltd.
[0126] Table 1 HIV-1 viral reverse transcriptase gene segment amplification primers
[0127]
Primer number
Primer sequence (5'-3')
PMV-001
gagagcttcaggtctgggg
PMV-002
CTGTTAGTGCTTTGGTTCCTCT
PMV-01...
Embodiment 2
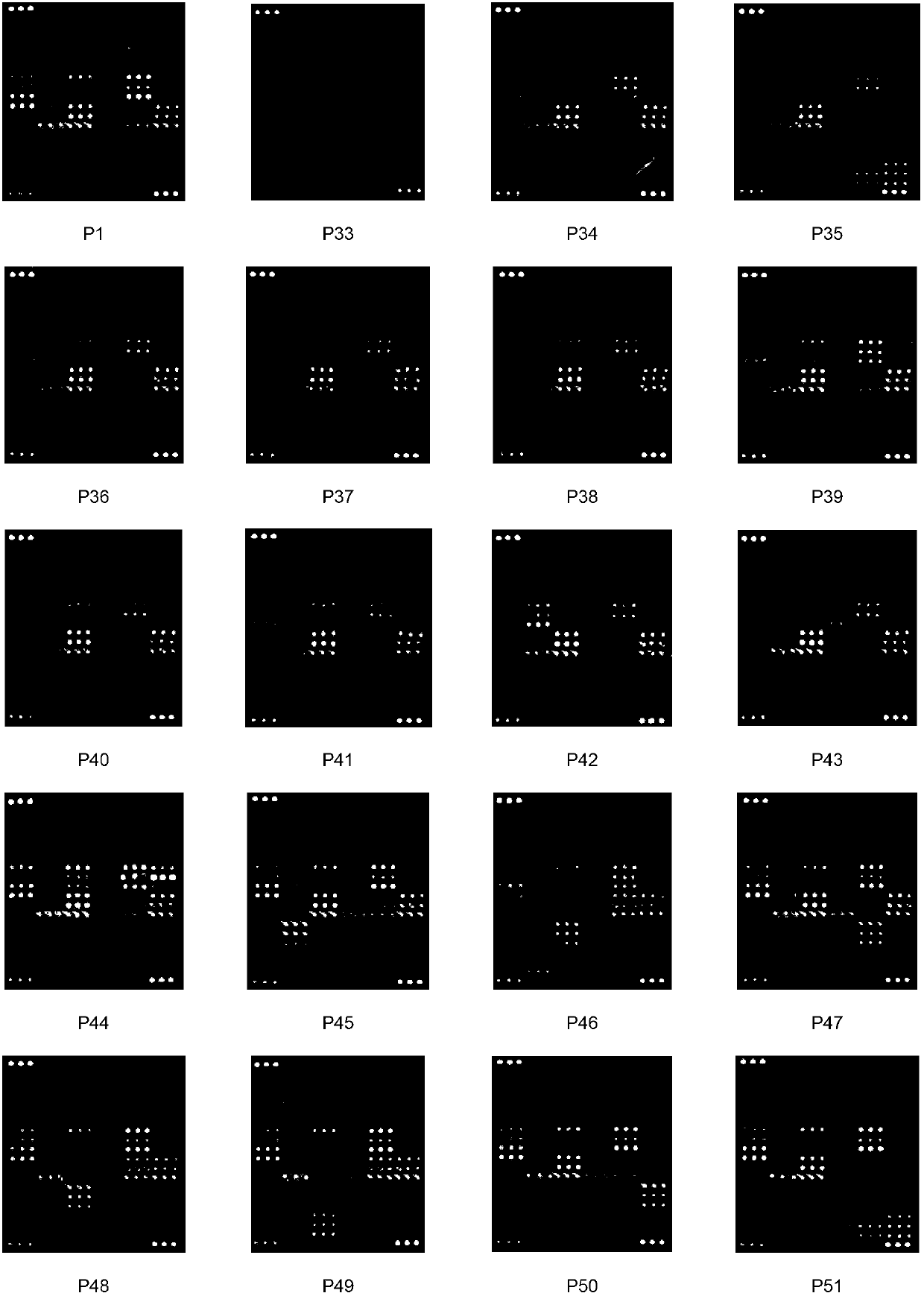
[0191] Example 2: Using the present invention to detect HIV-1 virus nucleoside reverse transcriptase inhibitor multi-site and low-abundance drug-resistant mutations
[0192] This example illustrates that the method and kit provided by the present invention can simultaneously detect samples containing multiple nucleoside reverse transcriptase inhibitor resistance mutations of HIV-1 virus.
[0193] This example also shows that the method provided by the present invention can detect drug-resistant mutations with an abundance as low as 2% contained in the sample, and is a method with extremely high sensitivity.
[0194] 1. Preparation of Oligonucleotide Primers and Probes
[0195] Method and steps are the same as in Example 1.
[0196] 2. Preparation of HIV-1 Virus Nucleoside Reverse Transcriptase Inhibitor Resistance Mutation Detection Chip
[0197] Method and steps are the same as in Example 1.
[0198] 3. Preparation of samples to be tested
[0199] The samples to be tested...
Embodiment 3
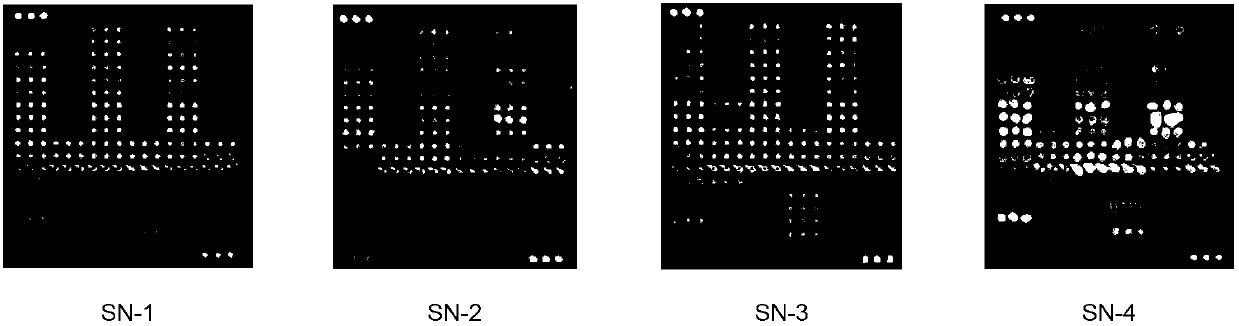
[0220] Example 3: Using the present invention to detect drug-resistant mutations of HIV-1 virus nucleoside reverse transcriptase inhibitors in plasma, whole blood and nucleic acid samples
[0221] This example illustrates that the method and kit provided by the present invention can be used to detect HIV-1 viral nucleoside reverse transcriptase inhibitor resistance mutations in plasma, whole blood and nucleic acid samples.
[0222] 1. Preparation of Oligonucleotide Primers and Probes
[0223] Method and steps are the same as in Example 1.
[0224] 2. Preparation of HIV-1 Virus Nucleoside Reverse Transcriptase Inhibitor Resistance Mutation Detection Chip
[0225] Method and steps are the same as in Example 1.
[0226] 3. Preparation of samples to be tested
[0227] In this embodiment, clinically collected whole blood (WB-6-8), plasma (XJ-6-7) and nucleic acid (R-5-6, D-3) samples were tested. After high-throughput sequencing, whole blood sample WB-6 contained nucleoside rev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com