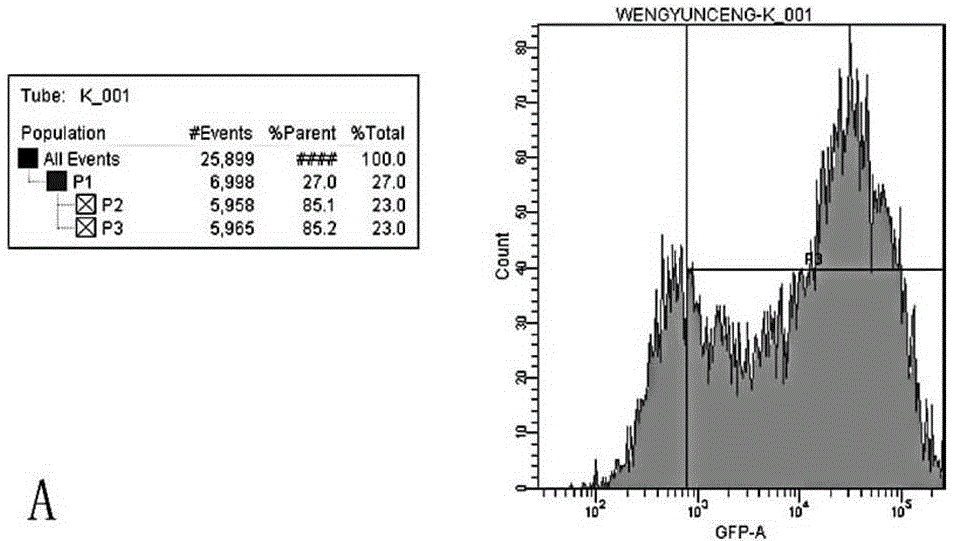
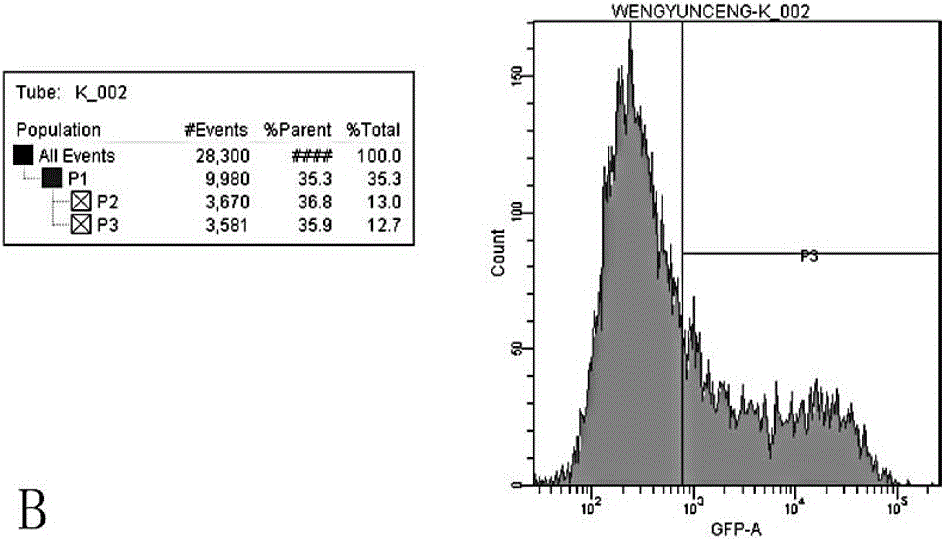
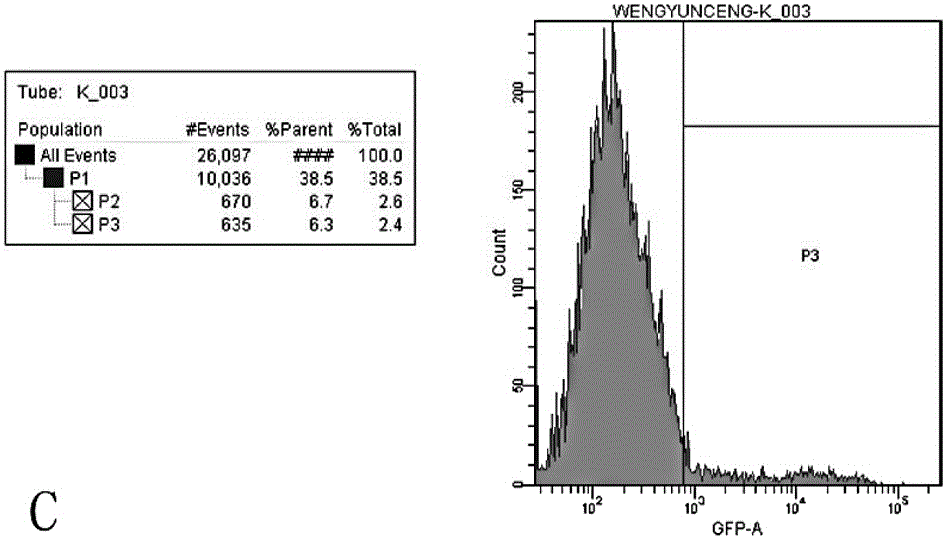
Application of recombinant lentivirus to HIV (human immunodeficiency virus) phenotypic drug resistance detection
A recombinant lentivirus and phenotype technology, applied in the field of medical molecular biology, can solve the problems of biosafety and high cost, and achieve the effect of strong versatility, safety, high efficiency and toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] The present invention will be described in detail below in conjunction with the accompanying drawings and embodiments.
[0040] A recombinant lentiviral system of the present invention is constructed by the following method, the steps are:
[0041] (1) Construction of transfer plasmid
[0042] The transfer plasmid of the present invention has double reporter gene luciferase Luciferase and ZsGreen, and its specific structure is as follows figure 1 shown. Luciferase and ZsGreen are independently expressed proteins, not fusion proteins.
[0043] Such as figure 2 As shown, the construction method of the transfer plasmid is as follows:
[0044] (1.1) Use BamHI and KpnI to double digest the pMD2.G plasmid, recover the digested product, and collect the 119bp CMV fragment;
[0045] (1.2) The pHAGE-EF1α-IRES-ZsGreen plasmid was double digested with BamHI and KpnI, and the 4736bppHAGE-IRES-ZsGreen fragment was recovered;
[0046] (1.3) Ligate the recovered CMV fragment and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com