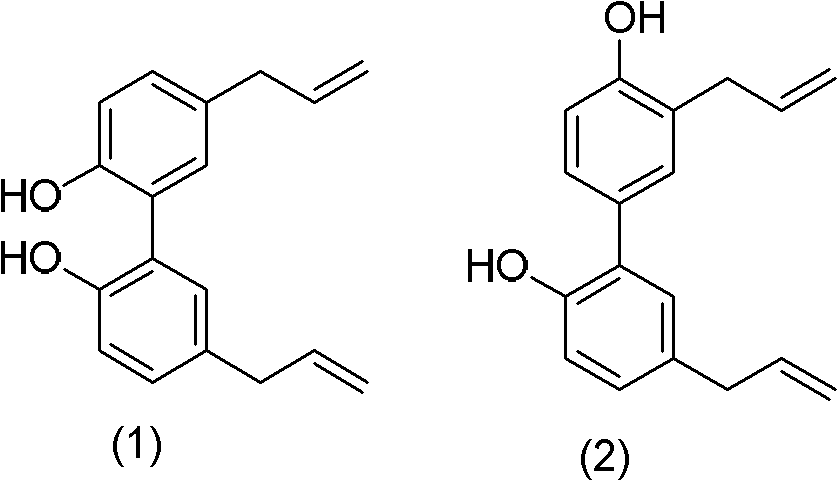
Application of cortex magnolia officinal extract in preparing medicine for healing and preventing acquired immune deficiency syndrome
An extract, AIDS technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 HIV-1 protease inhibitor activity detection method
[0023] Fluorescence resonance energy transfer (FRET) technology was used to measure the activity of inhibitors against HIV-1 protease, and the substrate was designed according to the recognition site of HIV-1 protease: MCA-gama-abu-Ser-Gln-Asn- Tyr-Pro-Ile-Val-Gln-Glu-Lys-Dnp, when the inhibitor has no effect on HIV-1 protease, HIV-1 protease can cleave the substrate, and the quencher (Dnp) is away from the fluorescent group (MCA ), the fluorophore absorbs a wavelength of 320nm and excites a wavelength of 405nm, and the fluorescence intensity is detected by a detector. If the inhibitor has an effect on HIV-1 protease, the quenching group will not be cut off, and the fluorophore absorbs a wave of 320nm The latter part of the energy will be transferred to the quenching group, and the intensity of the excitation wave at 405nm will be weakened, so as to detect the inhibitory effect of the inhibitor on HIV-1 prot...
Embodiment 2
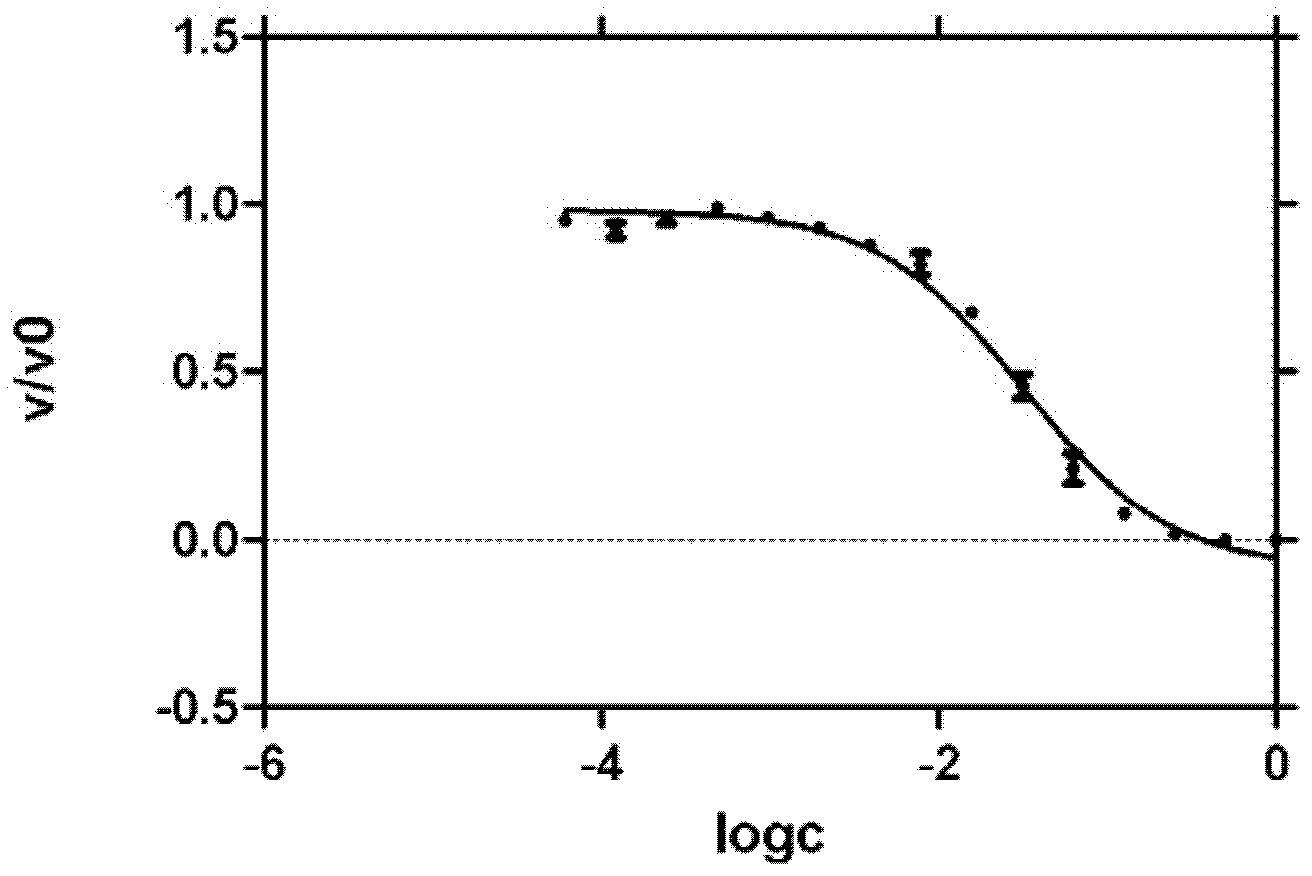
[0024] Example 2 IC 50 Value determination method
[0025]Prepare a high-concentration solution (generally 200uM) of the compound to be tested, and then perform a 1 / 2 concentration gradient dilution to form a series of concentration solutions. Generally, the activity at 12 concentrations is determined, and then the remaining activity corresponding to each compound concentration is calculated. Use the software GraphPad Prism 5 software analysis, make the curve of residual activity value and the logarithmic value of compound concentration, calculate IC 50 value.
Embodiment 3
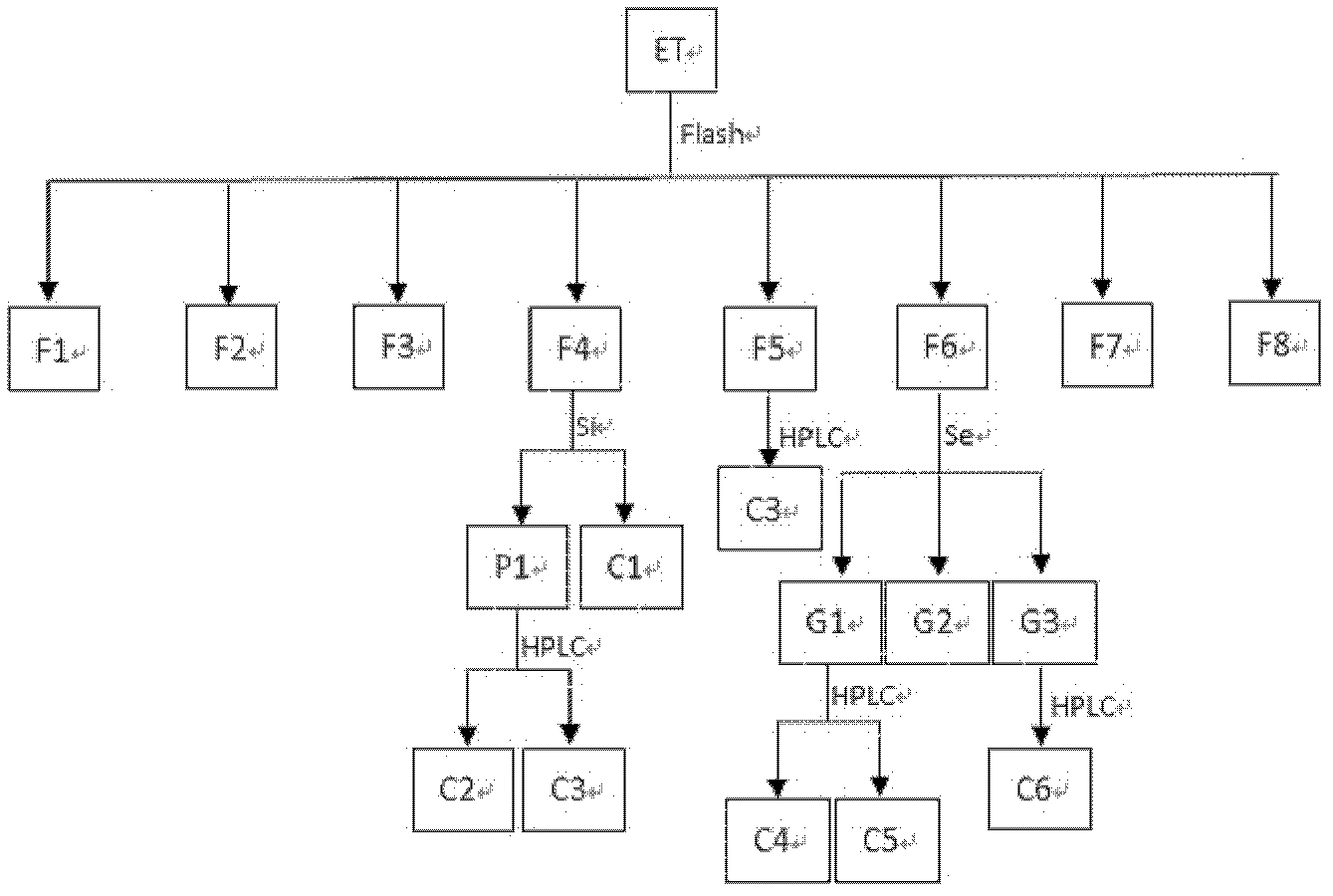
[0026] Example 3 The method for separating and purifying HIV-1 protease inhibitors from Magnolia officinalis
[0027] 1) Extraction and extraction of Magnolia officinalis medicinal materials
[0028] Take 15 kg of Magnolia officinalis as raw material, extract with 95% ethanol at 60°C, add 5 liters of 95% ethanol to each kg of Magnolia officinalis, and extract three times, and the obtained extract is filtered through gauze and then concentrated to obtain the extract. paste, the extract was dissolved into a suspension with a small amount of water, and three times the volume of ethyl acetate was added to extract three times to obtain the ethyl acetate extract, and the n-butanol extract was obtained in the same way, and the remaining components were concentrated to dryness into the water phase.
[0029] 2) Test the inhibitory activity of ethyl acetate and n-butanol extract to HIV-1 protease
[0030] Test the inhibitory effect of ethyl acetate extract and n-butanol extract to HIV-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com