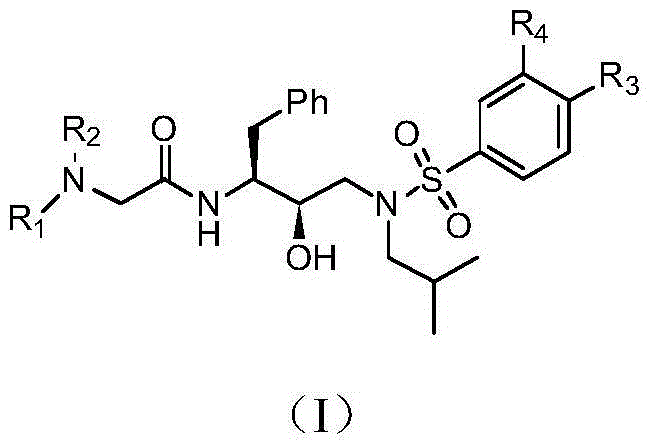
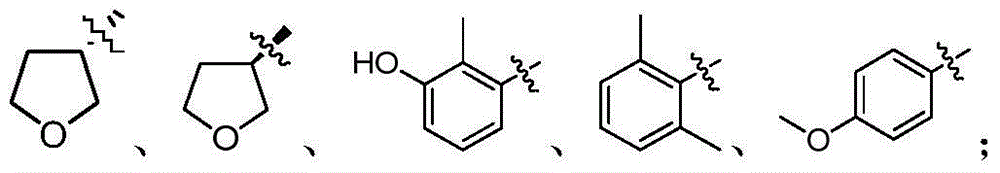
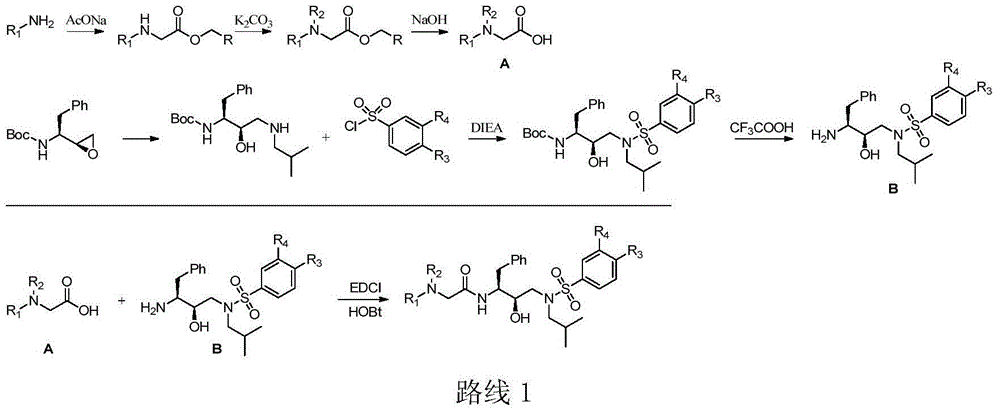
Tertiary amine analogical peptide derivative and application of tertiary amine analogical peptide derivative in inhibiting HIV-1 protease
A technology of derivatives and tertiary amines, used in the inhibition of HIV-1 protease, the preparation of tertiary amine derivatives can solve the problems of metabolic inactivation, AIDS, and increased blood drug concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] "Example 1" Preparation of Compound 1
[0159]
[0160] 2-((2,6-Dimethylphenyl)(2-morpholine-2-oxoethyl)amino)-N-((2S,3R)-3-hydroxy-4-(N-isobutyl Base-4-methoxyphenylsulfonamido)-1-phenylbutan-2-yl)-acetamide
[0161] Preparation of step A intermediate 2-(2,6-dimethylphenyl)aminoacetate
[0162]
[0163] Add 2,6-dimethylaniline (9.9g, 81.30mmol), ethyl chloroacetate (10.39mL, 97.56mmol), sodium acetate (8.0g, 97.56mmol) into an eggplant-shaped bottle containing 30mL of ethanol, and reflux for 3 Hour. The completion of the reaction was detected by TLC, the ethanol was removed by concentration under reduced pressure, the residue was extracted with ethyl acetate (50×3 mL), the organic phase was dried over anhydrous sodium sulfate, and concentrated to obtain a crude product. The crude product was purified by flash column with ethyl acetate-n-hexane (1:8) to obtain the target product (11.7 g, 69.2%) as brown oil.
[0164] 1 H NMR (400MHz, DMSO) δ6.92 (d, J = 7.4Hz...
Embodiment 2
[0188] "Example 2" Preparation of Compound 5
[0189]
[0190] 2-((2,6-Dimethylphenyl)(2-N,N-Dimethyl-2-oxoethyl)amino)-N-((2S,3R)-3-Hydroxy-4- (N-isobutyl-4-methoxyphenylsulfonamido)-1-phenylbutan-2-yl)-acetamide
[0191] Preparation of intermediate ethyl 2-((2,6-dimethylphenyl)(2-N,N-dimethyl-2-oxoethyl)amino)acetate
[0192]
[0193] According to the synthesis method of Step B in Example 1, react 2-(2,6-dimethylphenyl)aminoacetate and N,N-dimethylbromoacetamide at 100°C in the presence of potassium carbonate 1.5h prepared.
[0194] 1 H NMR (400MHz, CDCl 3 )δ7.01(dd, J=3.8,3.2Hz,3H),6.95(dd,J=8.5,6.2Hz,1H),4.13(q,J=7.1Hz,2H),4.04(s,2H), 4.01(s,2H),3.86(s,1H),3.03(s,1H),2.94(s,1H),2.91(s,3H),2.85(s,3H),2.46(s,6H),2.39 (s, 2H), 1.23 (t, J=7.1Hz, 3H); LC-MS (ESI, M+Na + ) m / z 315.2.
[0195] Preparation of intermediate 2-((2,6-dimethylphenyl)(2-N,N-dimethyl-2-oxoethyl)amino)acetic acid
[0196]
[0197] According to the synthesis method of step C in Example 1,...
Embodiment 3
[0202] 《Example 3》The preparation of compound 6
[0203]
[0204] 2-((2,6-Dimethylphenyl)(2-N,N-Dimethyl-2-oxoethyl)amino)-N-((2S,3R)-3-Hydroxy-4- (N-isobutyl-4-nitrophenylsulfonamido)-1-phenylbutan-2-yl)-acetamide
[0205] Preparation of intermediate (2S,3R)-1-benzyl-2-hydroxy-3-(N-isobutylamine-4-nitrosulfonamide)carbamate tert-butyl ester
[0206]
[0207] According to the preparation method of step E in Example 1, ((2S,3R))-1-benzyl-2-hydroxyl-3-(isobutylamine) tert-butyl carbamate and 4-nitrobenzenesulfonyl chloride were Prepared by stirring at room temperature for 3.5h under the catalyst of DIEA.
[0208] Melting point 113–115°C. 1 H NMR (400MHz, DMSO) δ8.37 (d, J = 8.8Hz, 2H), 8.06 (d, J = 8.8Hz, 2H), 7.25–7.12 (m, 5H), 6.69 (d, J = 8.9Hz ,1H),4.96(d,J=6.5Hz,1H),3.51–3.43(m,2H),3.38–3.35(m,1H),3.15(dd,J=13.6,8.4Hz,1H),3.08( dd,J=14.9,8.8Hz,1H),2.96–2.92(m,2H),2.03–1.93(m,1H),1.25(s,9H),0.85(d,J=6.7Hz,3H),0.83 (d, J=6.7Hz, 3H); LC-MS (ESI, M+H + ) m / z 521.9....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com