Process for the preparation of (omega-aminoalkylamino)alkyl halides and conversion to amifostine
A technology of aminoalkylamino and alkyl halide dihydrohalides, which is applied in the field of preparation of alkyl halides, and can solve the problems of not reaching the ideal output and long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
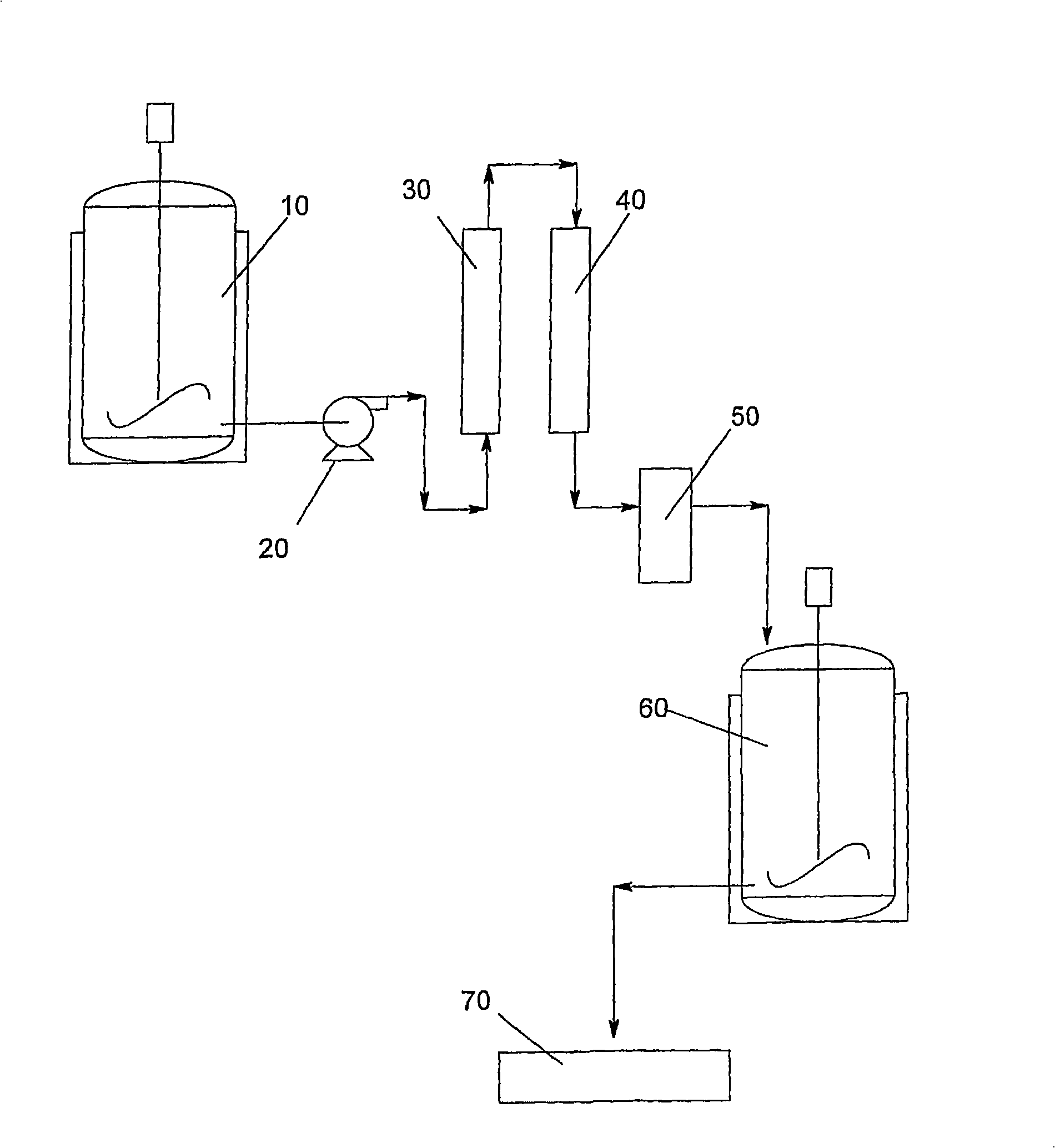
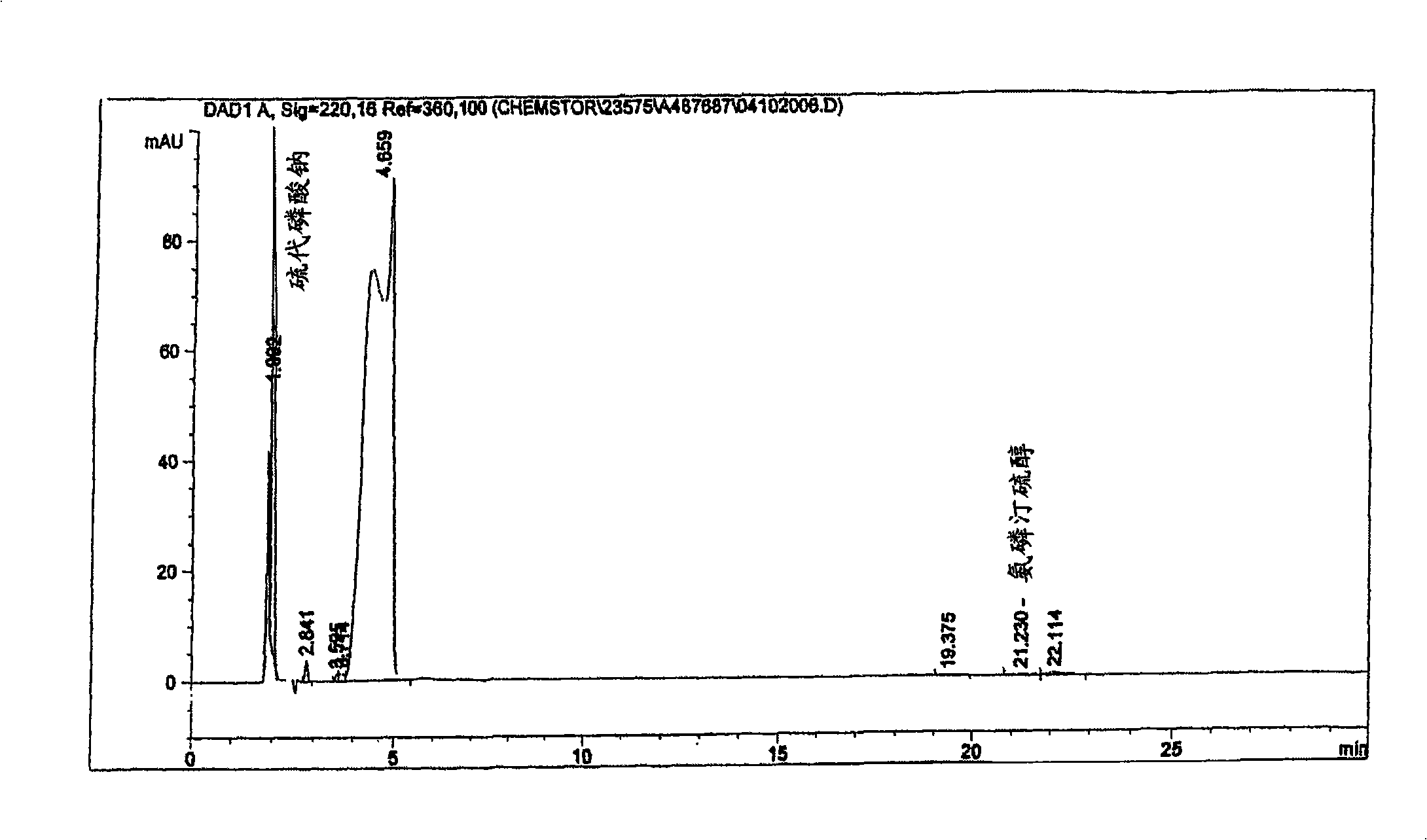
[0047] B. Preparation and purification of amifostine monohydrate and amifostine trihydrate
[0048] In another aspect of the present invention, (ω-aminoalkylamino)alkyl halide dihydrohalides of formula (III) can be used to prepare different synthetic products. For example, compounds of formula (III) can be used in the manufacture of useful medical compounds such as amifostine (Ethyol ) including a large number of cell protection / radiation protection agents. These compounds are generally called "S-ω-(ω-aminoalkylamino)alkyl dihydrogenphosphorylsulfonyl" (formula IV), and can be synthesized according to the method in scheme II:
[0049]
[0050] Scheme II
[0051] According to this process, a compound of general formula (III), such as 2-(3-aminopropylamino)ethylbromodihydrobromide, can be contacted with sodium thiophosphate sufficient to form a compound of formula (IV) and a hydrate thereof. a period of time.
[0052] Crude phosphorothioate compounds of formula (IV) su...
example 1
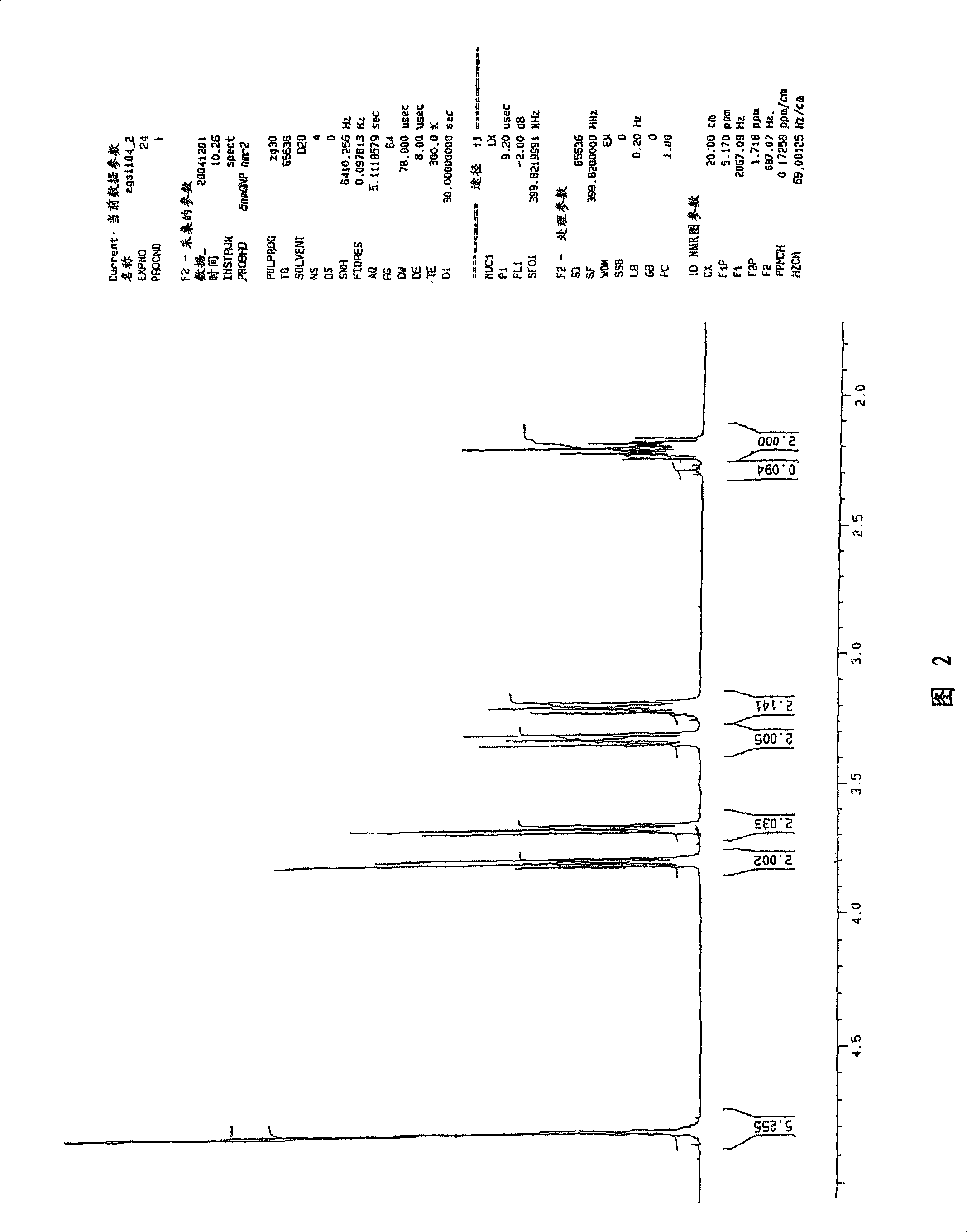
[0058] Example 1: Laboratory preparation of 2-(3-aminopropylamino)ethyl bromide dihydrobromide
[0059] In a nitrogen-purged glove box, a 3-liter four-neck round bottom flask equipped with mechanical stirring, thermocouple, nitrogen inlet connector, and septum was charged with 2-(3-aminopropylamino)ethanol ( 144 g; 1.22 mol) and sulfolane (1.00 L; 10.5 mol). Move this to a fume hood where the nitrogen line is connected, replaced by bulkheads to support 1 / 8 inch diameter Teflon Connectors for tubing (to connect with HBr fine compressed gas cylinders). While stirring, hydrogen bromide (HBr) gas was introduced below the liquid surface at a rate that allowed the temperature to rise to about 130°C. After the exotherm had ceased and HBr had ceased to be taken up, the addition was stopped; two equivalents had reacted, yielding the dihydrobromide salt of the starting alcohol. Replace the connector on the reaction flask with an isobaric dropping funnel containing phosphorus tribrom...
example 2
[0061] Example 2: Synthesis of sodium thiophosphate
[0062] Prepare sodium thiophosphate and its hydrate according to the method described in the literature [Inorganic Synthesis, 5:102 (1957); ibid.17:193 (1997)]. reaction. The only difference is that phosphorus trichloride sulfur is slowly added to the caustic solution at reflux in order to control the exothermic reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com