Stable amorphous amifostine compositions and methods for the preparation and use of same
An amifostine and amorphous technology, which is applied in the directions of active ingredients of phosphorus compounds, pharmaceutical combinations, medical preparations of non-active ingredients, etc., can solve the problems of undisclosed amorphous heat-stable amifostine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0096] Stable amorphous amifostine dosage form containing nicotinamide and PVP
[0097] At 25°C, 100 mg / mL amifostine, 12.5 mg / mL nicotinamide (Aldrich) and 10 mg / mL polyvinylpyrrolidone 30 (PVP30: BASFAktiengesellschaft, Feinchemie, 0-6700 Ludwigshafen, Germany) The aqueous solution was sterilized and filtered, and then divided into 5 mL aliquots, each of which was transferred to a 10 mL vial. Place the freeze-drying stopper in the vial and place the sample on the shelf of the freeze dryer to maintain at 5°C. Reduce the shelf temperature to -45°C within 60 minutes and keep it at this temperature for about 3 hours. Then the condenser of the freeze dryer was opened and the chamber was evacuated to approximately 100 μmHg. After the chamber vacuum has reached equilibrium, the shelf temperature is increased to -25°C within 60 minutes, and the vacuum degree is kept constant during the period. Keep the shelf temperature at -25°C for approximately 12 hours. The shelf temperature was then...
Embodiment 3
[0100] Determination of crystallinity
[0101] The crystallinity of the dosage form of the present invention can be determined by powder x-ray diffraction, as described, for example, in Remington's Pharmacy, 18th edition, page 173; United States Pharmacopeia, 23rd edition (1995), 1843-1844.
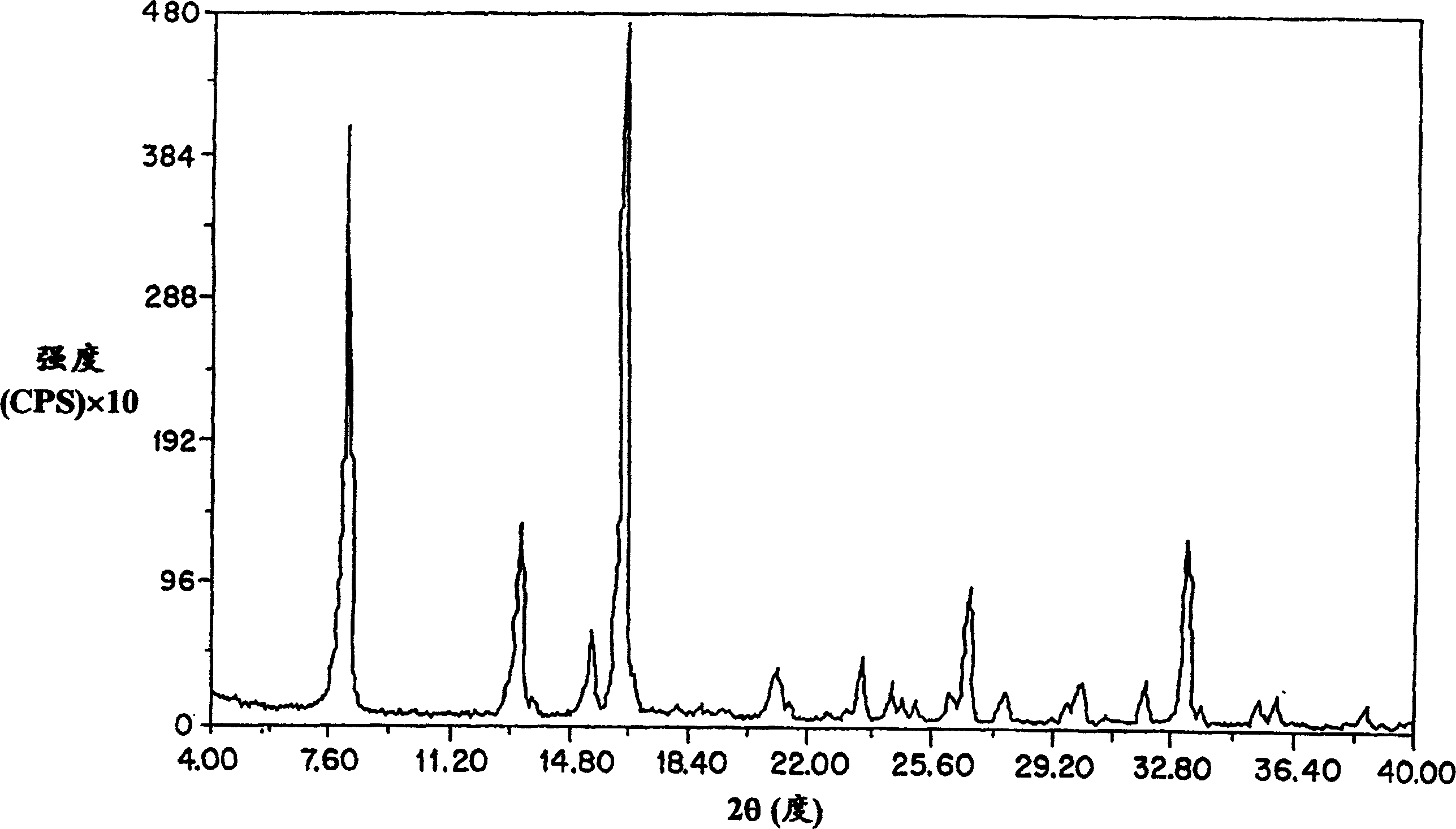
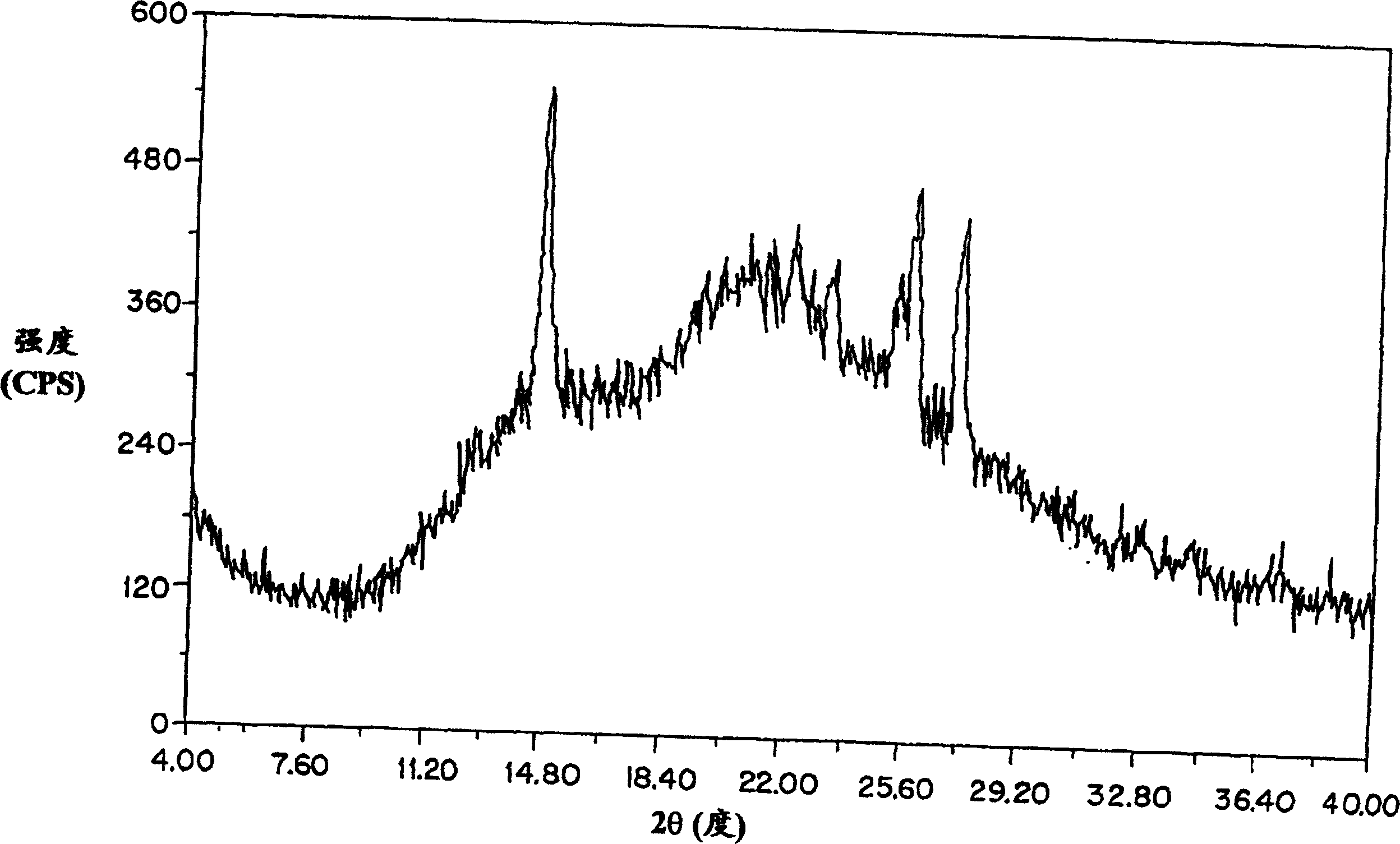
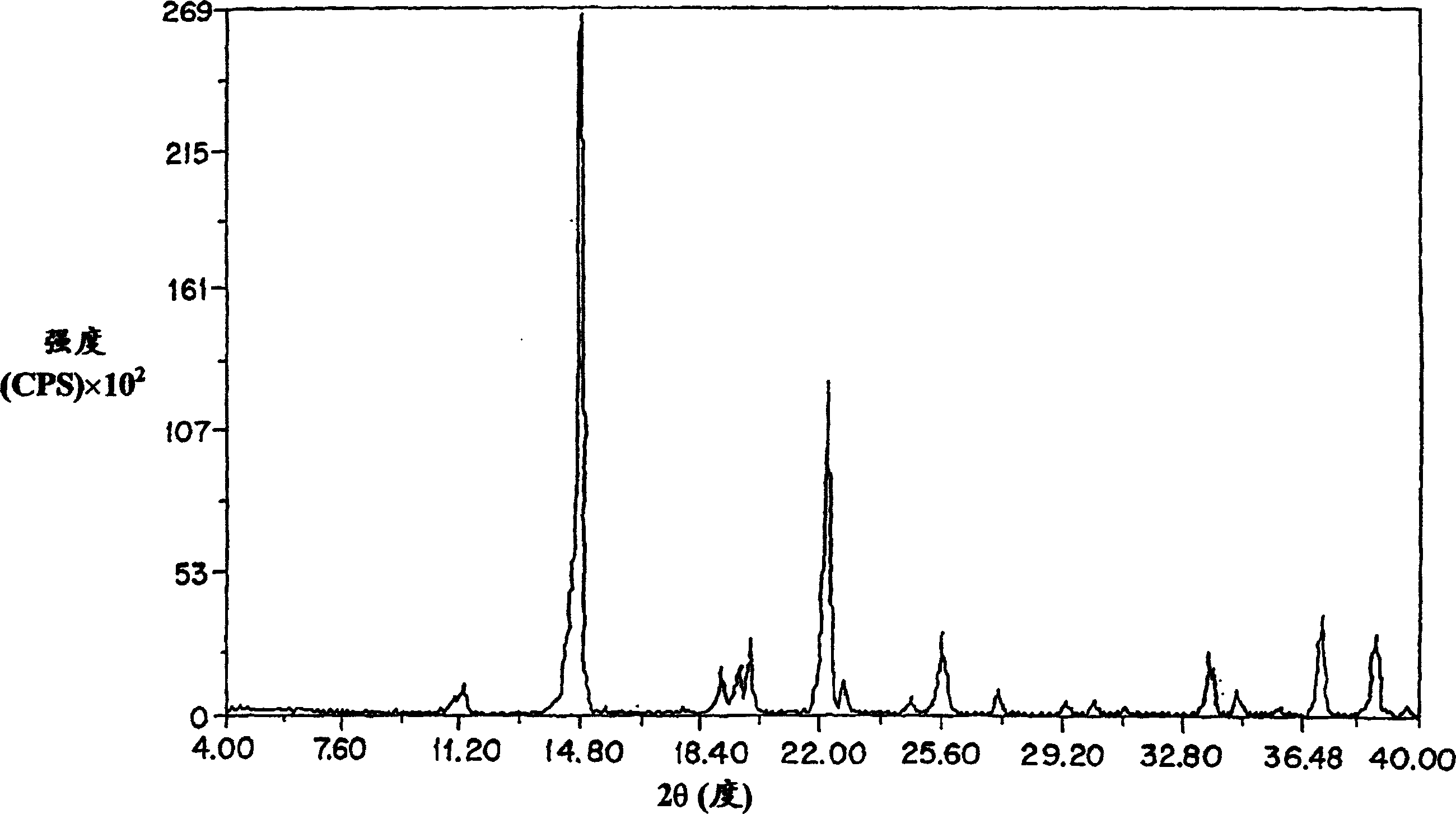
[0102] figure 2 The general powder x-ray diffraction spectrum of the amorphous amifostine dosage form prepared according to the method of Example 1 is shown, measured with a Geiger-Muller detector using nickel-filtered CuKα radiation. The diffraction pattern contains the broad baseline nature of the amorphous material. The peaks at 2θ≈14.8, 25.6, and 26.3 are due to nicotinamide and / or noise. From image 3 From a point of view, the arrangement is clear. It shows the x-ray powder diffraction pattern of crystalline nicotinamide.
[0103] Figure 4 Shows the difference between the x-ray diffraction pattern of crystalline amifostine prepared as described by US Patent No. 5,591,731 and the amorp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com