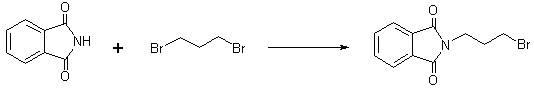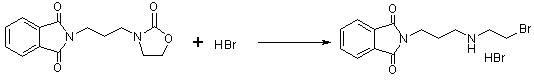Method for preparing amifostine
A technology of amifostine and amino group is applied in the preparation field of adjuvant therapeutic agent amifostine, which can solve the problems of industrialized production obstacles, difficult long-distance transportation, feasibility limitation and the like, and achieves the effects of low price, safe use and simplified production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] a) Add phthalimide (147g, 1.00 mol), N,N-dimethylformamide (DMF) 700mL, triethylamine ( 101g, 1.00 mol), after the addition, control the temperature of the reaction solution at 0°C to 5°C, start to slowly add 1,3-dibromopropane (202g, 1.00 mol), and control the temperature of the reaction solution at 10°C to Stir at 15°C for 3 hours. After the reaction was completed, concentrated under reduced pressure to recover N,N-dimethylformamide (DMF), added 500 mL of water to the concentrate, and solids were precipitated, filtered, and the filter cake was recrystallized once with 90% ethanol, dried in vacuo at 40°C to obtain N-( 3-Bromopropyl) phthalimide weighed 268g (1.00 mol), measured the melting point of 72°C to 75°C, and the yield was 100%.
[0062] b) In a reaction flask equipped with stirring and a thermometer, add N-(3-bromopropyl)phthalimide (268g, 1.00mol) and 600mL of acetonitrile prepared in the previous step to control the temperature of the reaction solution Slow...
Embodiment 2
[0069] a) Add phthalimide (147g, 1.00 mol), dimethyl sulfoxide 700mL, and sodium carbonate (53g, 0.5 mol) to the reaction flask equipped with stirring, thermometer and dropping device. , control the temperature of the reaction solution at 0° C. to 5° C., start to slowly add 1,3-dibromopropane (404 g, 2.00 mol) dropwise, and then control the temperature of the reaction solution at 1° C. to 5° C. and stir for 5 hours. After the reaction was completed, concentrated under reduced pressure to recover dimethyl sulfoxide, added 500 mL of water to the concentrate, and solids were precipitated, filtered, and the filter cake was recrystallized once with 90% ethanol, and dried under vacuum at 40°C to obtain N-(3-bromopropyl) o-phenyl Dicarboximide weighs 268g (1.00 mol), and its melting point is 72°C to 75°C. The yield is 100%.
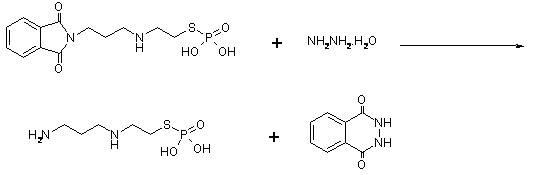
[0070] b) In a reaction flask equipped with stirring and a thermometer, add N-(3-bromopropyl)phthalimide (268g, 1.00mol) prepared in the previous step reaction,...
Embodiment 3
[0077] a) Add phthalimide (147g, 1.00mol), dioxane 700mL, and sodium methoxide (1.5mol) into a reaction flask equipped with a stirring, thermometer, and dropping device. After the addition, control the reaction The liquid temperature was 0°C-5°C, and 1,3-dibromopropane (202 g, 1.00 mol) was slowly added dropwise. After the dropwise addition, the reaction liquid temperature was controlled at 5°C-10°C and stirred for 4 hours. After the reaction was completed, the dioxane was concentrated under reduced pressure to recover dioxane, the concentrate was added with 500 mL of water, and a solid precipitated out, filtered, and the filter cake was recrystallized once with 90% ethanol, and dried under vacuum at 40°C to obtain N-(3-bromopropyl)phthalic dioxane Formimide weighed 268g (1.00 mol), measured melting point 72°C-75°C, yield 100%.
[0078]b) In a reaction flask equipped with stirring and a thermometer, add N-(3-bromopropyl)phthalimide (268g, 1.00mol) prepared in the previous step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com