PEGylation modified amifostine as well as preparation method and use of PEGylation modified amifostine
A technology for pegylated amifostine and polyethylene glycol, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of short action time, large side effects, and metabolic wait for the question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] This example is used for the synthesis of linear monofunctional polyethylene glycol (0.5kDa) amifostine
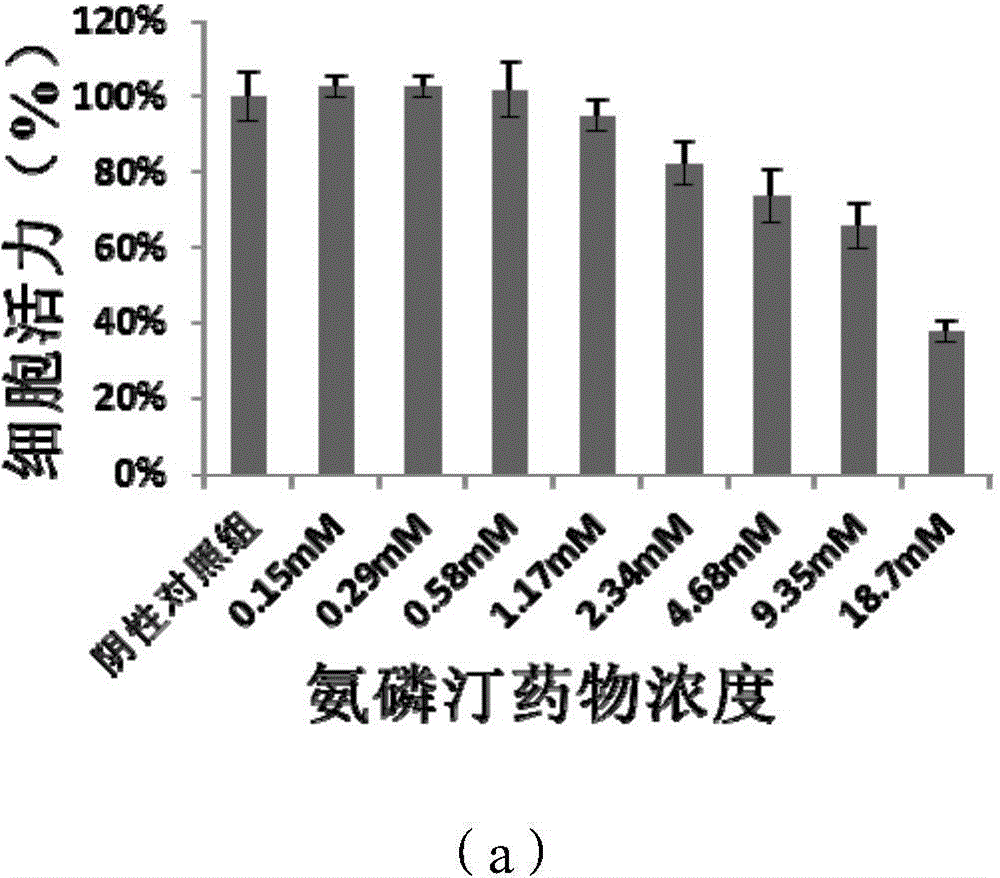
[0058] Linear monofunctional polyethylene glycol acetate succinimidyl ester (0.5kDa) (100mg, ≈200μmol) and amifostine trihydrate (43mg, ≈200μmol) were placed in a 50ml round bottom flask, and 10ml pH=6.0 phosphoric acid was added Salt buffer, react at 0℃ for 48h. After the reaction, the mixed solution was dialyzed in three-distilled water (pH 7-8) through a dialysis bag with a molecular weight cut-off of 300 Da. The dialysis process was carried out in a chromatography cabinet at 4°C. The three-distilled water was changed every 4 hours. Amifostine linked to ethylene glycol derivatives is eliminated during this process. The product after dialysis was freeze-dried at low temperature for 36 hours to obtain a powdered dry bulk drug with a yield of about 40%.
Embodiment 2
[0060] This example is used for the synthesis and purification of four-arm multifunctional polyethylene glycol (40kDa) amifostine.
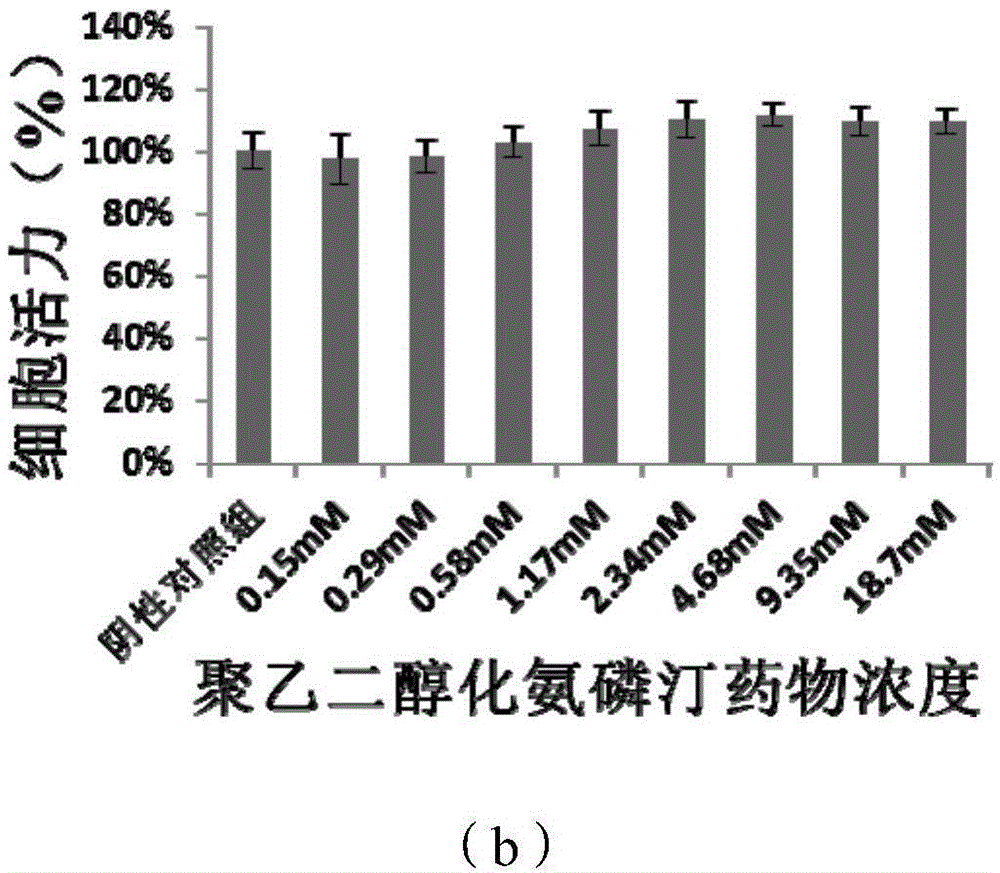
[0061] Four-arm polyethylene glycol acetate-N-succinimidyl ester (40kDa) (200mg, ≈5μmol) and amifostine trihydrate (43mg, ≈200μmol) are placed in a 10ml reaction flask and 2ml pH=10 phosphate buffer Liquid, react at 60°C for 0.5h. After the synthesis reaction is completed, the reacted mixed solution is freeze-dried at a low temperature for 36 hours to obtain a powdery dry product. Re-dissolve PEGylated amifostine with a molecular weight of about 40kDa in 40ml of dichloromethane. After shaking to dissolve, centrifuge at 12000rpm for 10min, take the supernatant and repeat the centrifugation step 4 times, then add it to 360ml of anhydrous ether Carry out recrystallization, centrifuge at 10,000 rpm for 10 minutes, wash the precipitated product with anhydrous ether three times, and place it in a vacuum drying oven at room temperature for drying to obtai...
Embodiment 3
[0063] This example is used for the synthesis of four-arm multifunctional polyethylene glycol (5kDa) amifostine.
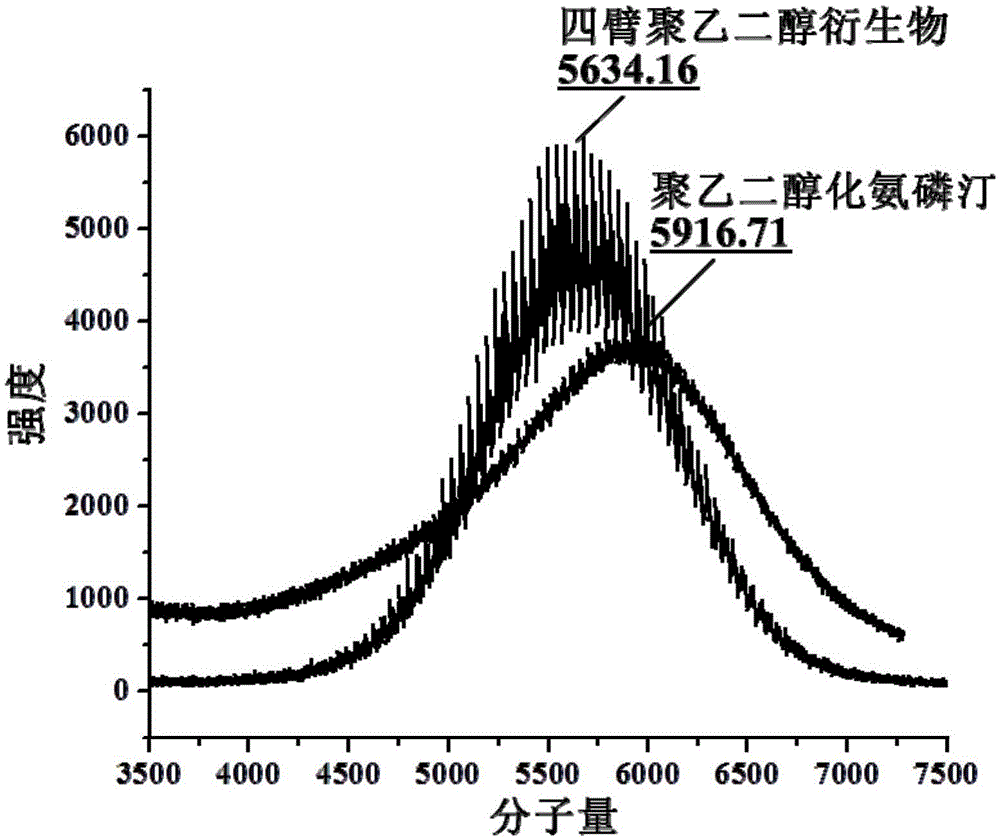
[0064] Four-arm polyethylene glycol acetate-N-succinimidyl ester (5kDa) (500mg, ≈95μmol) and amifostine trihydrate (125mg, 480μmol) were placed in a 50ml round bottom flask, and 10ml pH=7.4 phosphate was added Buffer, react at room temperature for 2h. After the reaction, the mixed solution was dialyzed in three-distilled water (pH 7-8) through a dialysis bag with a molecular weight cut-off of 1kDa. The dialysis process was carried out in a chromatography cabinet at 4°C. The three-distilled water was changed every 4 hours, and the Amifostine linked to ethylene glycol derivatives is eliminated during this process. The product after dialysis was freeze-dried at low temperature for 36 hours to obtain a powdery dry bulk drug. Compared with Examples 1 and 2, the structure of the amifostine linked to the polyethylene glycol derivative is stable and the product purity is h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com