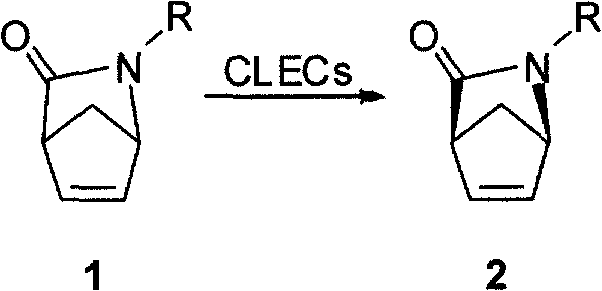
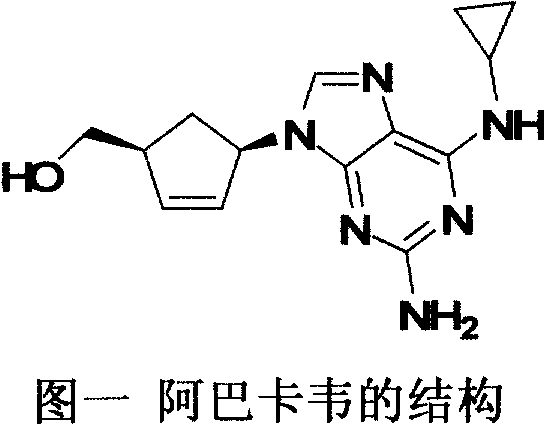
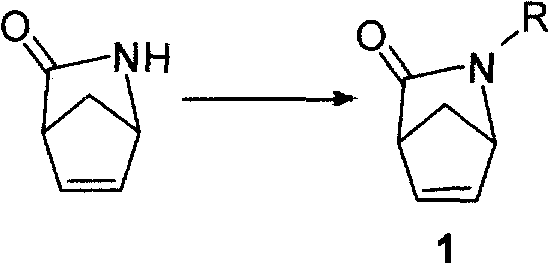Synthetic method for chiral carbocyclic ring intermediate of abacavir
A synthesis method and intermediate technology, applied in the field of enzymatic synthesis, can solve the problems of long synthesis steps, high synthesis cost, low yield and optical purity, etc., and achieve the effects of short reaction time, high yield and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Step 1: Add acetic anhydride (3.0 mol) dropwise to a THF solution (500 ml) of compound A (1.5 mol) and triethylamine (4.5 mol). With stirring, the system was heated to reflux for 23 hours. Activated carbon (37.5 g) was added, the system was stirred for 20 minutes, and filtered. The system was concentrated in vacuo, water (1000 ml) was added to the residue, and the aqueous phase was extracted 3 times with cyclohexane (1000 ml each). The organic phases were combined, washed once with saturated brine (500 mL), and dried over anhydrous sodium sulfate. The solvent was removed by rotary evaporation and dried under vacuum to obtain compound 1 as a light brown oil (yield 74%).
[0026] Step 2: Dissolve compound 1 (1 mole) in 1200 milliliters of ethanol, add the above-mentioned organic solution to the phosphate buffer solution of subtilisin cross-linking enzyme crystals (20 grams of wet weight subtilisin cross-linking enzyme crystals are suspended in In 1800 ml of...
Embodiment 2
[0028]
[0029] Step 1: Boc anhydride (3.0 mol) was added dropwise to a mixed solution of compound A (1.5 mol) and triethylamine (4.5 mol) in THF (250 ml) / water (250 ml). Under stirring, the system reacted overnight at room temperature. The system was concentrated in vacuo, water (1000 ml) was added to the residue, and the aqueous phase was extracted 3 times with ethyl acetate (1000 ml each). The organic phases were combined, washed once with saturated brine (500 mL), and dried over anhydrous sodium sulfate. The solvent was removed by rotary evaporation and dried under vacuum to obtain compound 3 as a light brown oil (yield 85%).
[0030] Step 2: Dissolve compound 3 (1 mole) in 1500 milliliters of isopropanol, add the above-mentioned organic solution to the phosphate buffer solution of subtilisin cross-linking enzyme crystals (20 grams of wet weight of subtilisin cross-linking enzyme crystals suspended in 1800 ml of phosphate buffer solution, pH equal to 8). Keep the sys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com