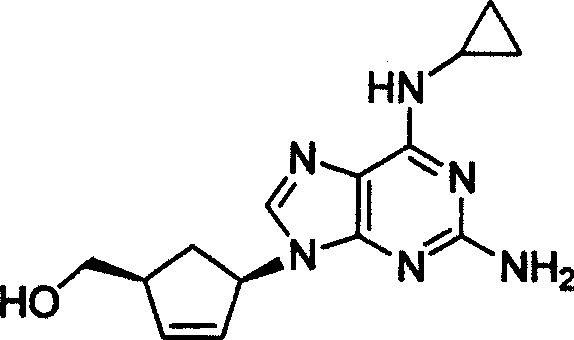
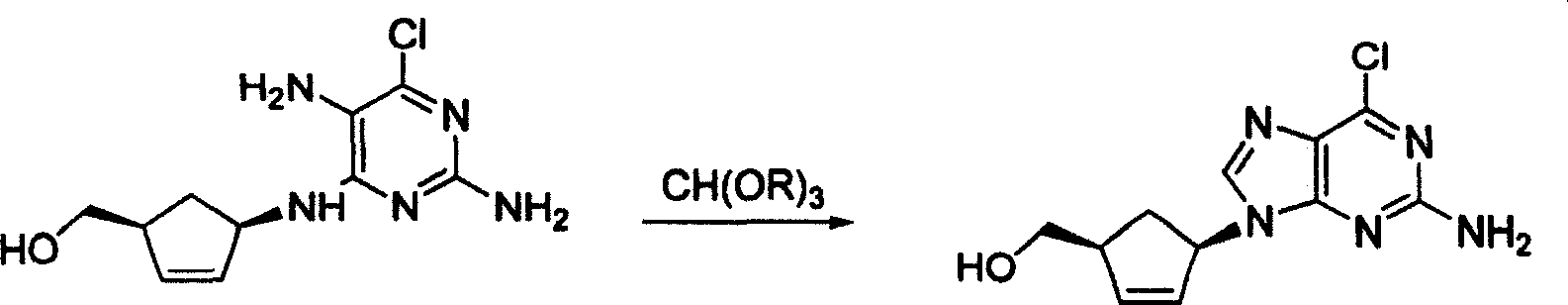A method for preparing optics pure abacavir
An optical and synthetic method technology, applied in the field of preparation of optically pure abacavir, can solve the problems of three wastes, complicated operation, etc., and achieve the effect of simple operation and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
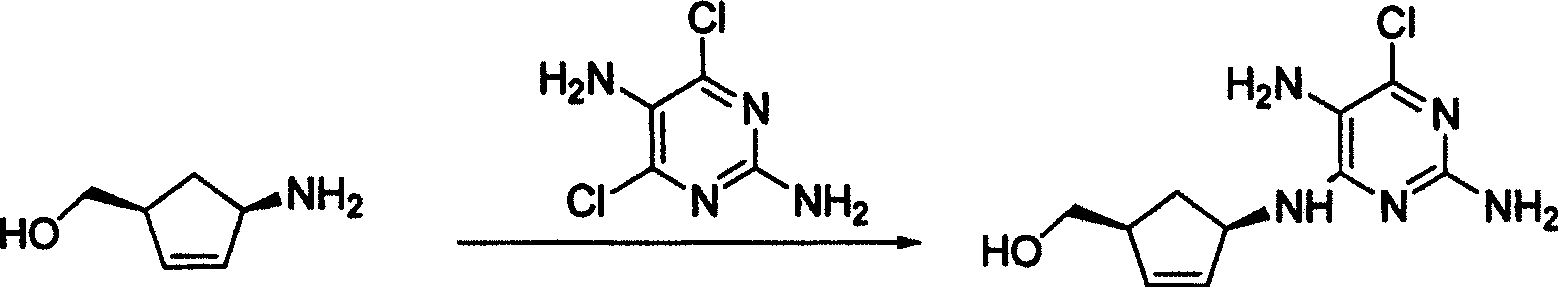
[0024] Embodiment 1 (1S, 4R)-4-[(2,5-diamino-6-chloro-4-pyrimidinyl) amino]-2-cyclopentenyl-1-methanol synthesis
[0025] (1S,4R)-4-amino-2-cyclopentenyl-1-methanol (113mg, 1mmol), 2,5-diamino-4,6-dichloropyrimidine (358mg, 2mmol), 1mL triethylamine , 10mL of methanol, heated to reflux, followed by spotting the plate until the end of the reaction, evaporated the solvent, and separated on a silica gel column to obtain a white flaky solid (1S, 4R)-4-[(2,5-diamino-6-chloro-4-pyrimidine Base)amino]-2-cyclopentenyl-1-methanol 109 mg, yield 43%.
Embodiment 2
[0026] Embodiment 2 (1S, 4R)-4-[(2,5-diamino-6-chloro-4-pyrimidinyl) amino]-2-cyclopentenyl-1-methanol synthesis
[0027] (1S,4R)-4-amino-2-cyclopentenyl-1-methanol (226mg, 2mmol), 2,5-diamino-4,6-dichloropyrimidine (179mg, 1mmol), 2mL triethylamine , 10mL of N,N-dimethylformamide, heated to reflux, followed by spotting the plate until the end of the reaction, evaporated the solvent, and separated on a silica gel column to obtain a white flaky solid (1S,4R)-4-[(2,5-diamino -6-Chloro-4-pyrimidinyl)amino]-2-cyclopentenyl-1-methanol 144 mg, yield 57%.
Embodiment 3
[0028] Embodiment 3 (1S, 4R)-4-[(2,5-diamino-6-chloro-4-pyrimidinyl) amino]-2-cyclopentenyl-1-methanol synthesis
[0029] (1S,4R)-4-amino-2-cyclopentenyl-1-methanol (113mg, 1mmol), 2,5-diamino-4,6-dichloropyrimidine (179mg, 1mmol), 1mL triethylamine , 10mL of dichloromethane, heated to reflux, followed by spotting the plate until the end of the reaction, evaporated the solvent, and separated on a silica gel column to obtain a white flaky solid (1S, 4R)-4-[(2,5-diamino-6-chloro-4 -pyrimidinyl)amino]-2-cyclopentenyl-1-methanol 71 mg, yield 28%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com