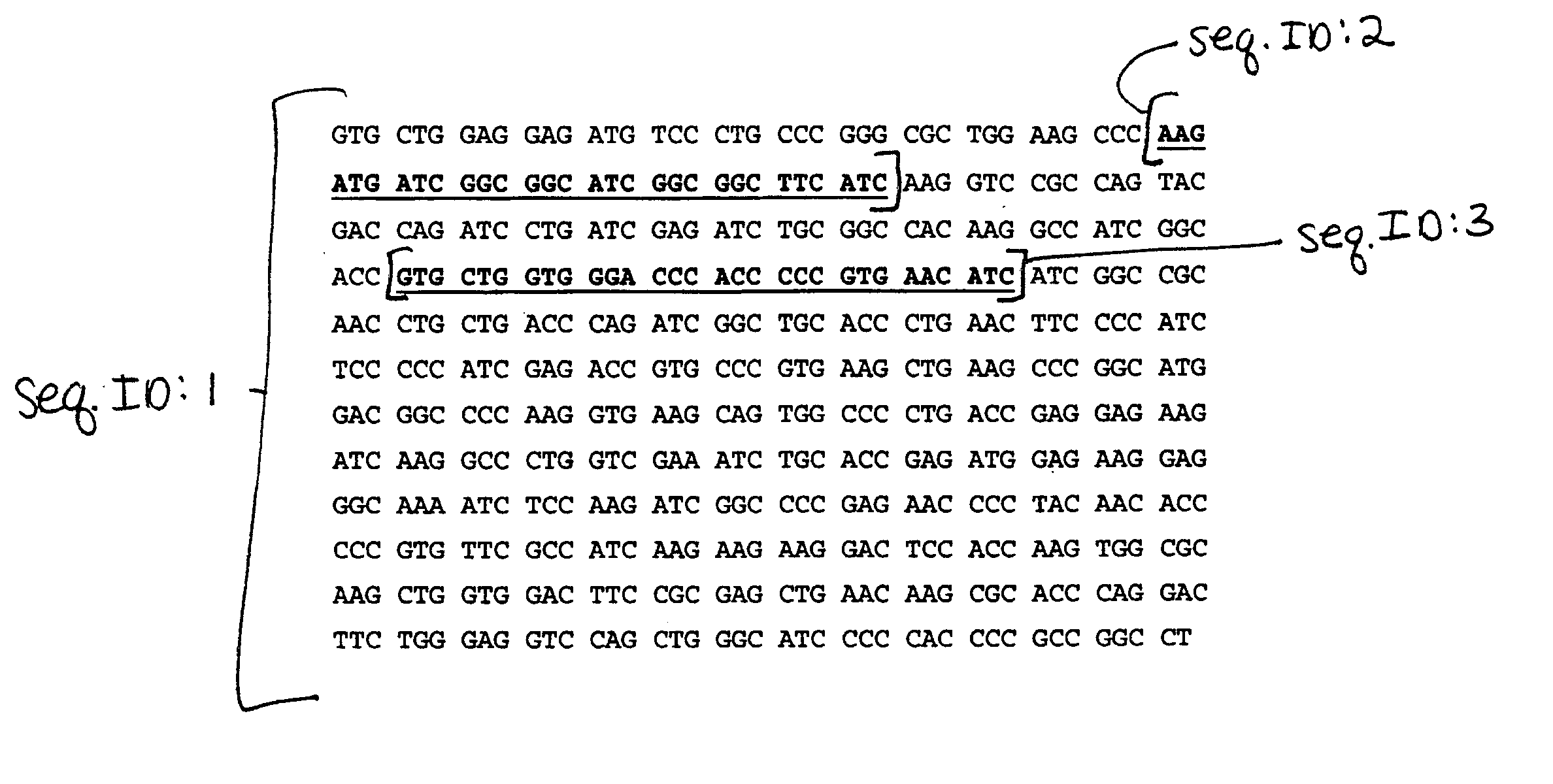Patents
Literature
42 results about "Hiv infected patients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HIV-infected patients are at an increased risk for developing Diabetes. More and more patients with HIV are controlling their disease well. When viral loads are under control, patients often are seen by clinicians for other problems. Diabetes seems to be one of those problems that get a lot of attention.
Non-nucleoside reverse transcriptase inhibitors
InactiveUS20080249131A1BiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorNon nucleoside inhibitor
Owner:ARDEA BIOSCIENCES INC
Non-nucleoside reverse transcriptase inhibitors
InactiveUS20060135556A1Reduce virus spreadSuppression problemBiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorNon nucleoside inhibitor
Various carbonyl amides are employed in vitro and in vivo as non-nucleoside inhibitors of a reverse transcriptase, and particularly of HIV reverse transcriptase. Therefore, contemplated compounds may be employed in the treatment of HIV infected patients. Further contemplated aspects include pharmaceutical compositions comprising therapeutically effective amounts of contemplated compounds.
Owner:ARDEA BIOSCI
Combined therapy for treatment of HIV infection
InactiveUS7094413B2Good curative effectReduce resistanceBiocideSugar derivativesImmunodeficiency virusGastrointestinal complications
The present invention relates to pharmaceutical preparations and methods for treating individuals infected with the human immunodeficiency virus (HIV). The pharmaceutical preparations comprise an immunomodulating agent and a anti-retroviral compound. The pharmaceutical preparations are used to treat HIV infected patients, particularly for gastrointestinal complications arising from viral infection. In addition, the pharmaceutical preparations of the present invention have the effect of raising the levels of CD4+ single positive and CD4+ and CD8+ double positive T cells, thus promoting restoration and normalization of the immune system following HIV infection.
Owner:SANGSTAT MEDICAL +1
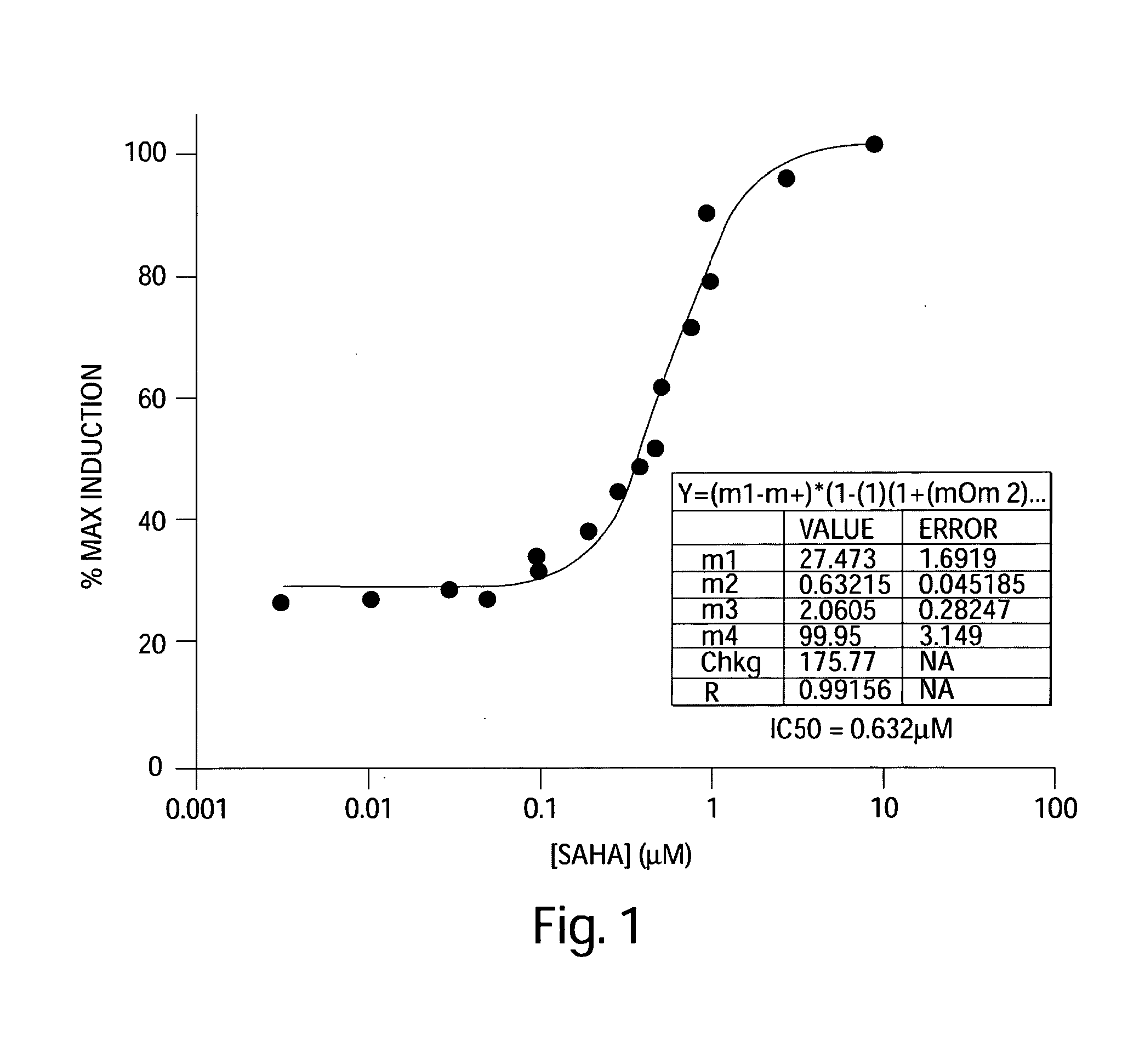
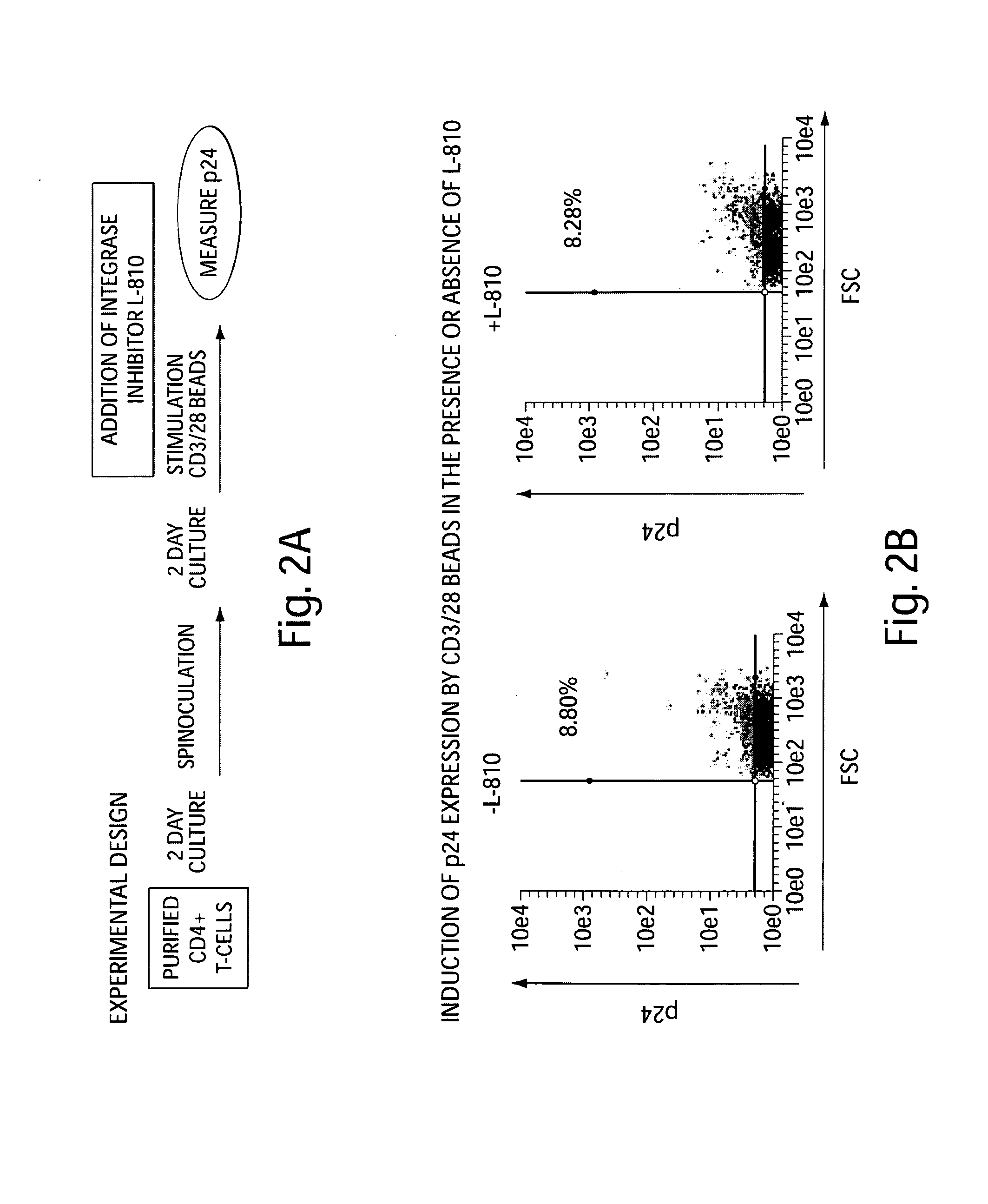
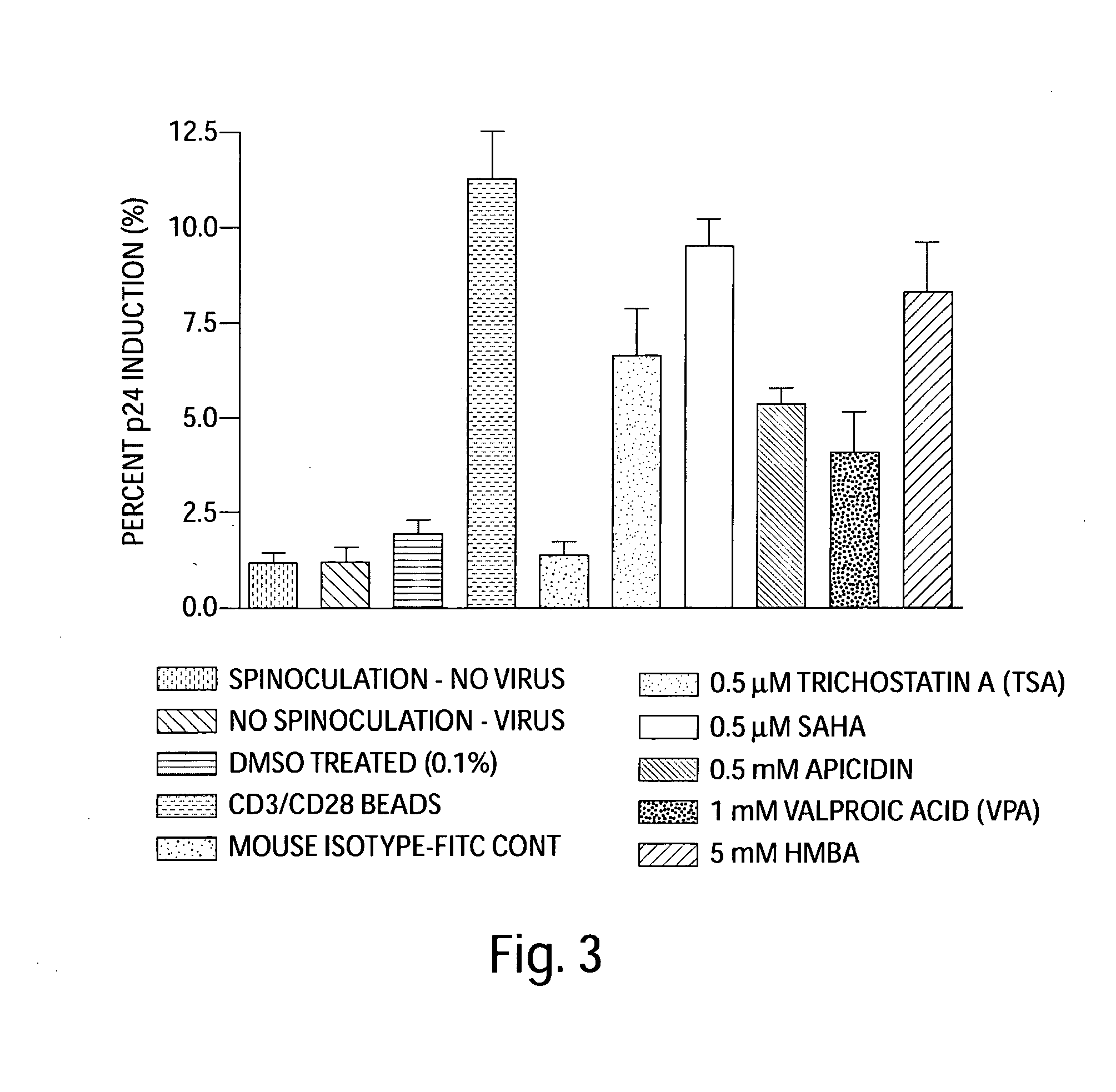
Methods of Using SAHA for Treating HIV Infection
The present invention relates to pharmaceutical preparations and methods for treating individuals infected with the human immunodeficiency virus (HIV). The pharmaceutical preparations comprise SAHA and another anti-viral agent. The invention also relates to methods for treating HIV infected patients, particularly patients with persistent, latent HIV infection of CD4+ T cells, and thus make it possible to not just suppress but to eradicate the HIV infection.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
Pharmaceutical compositions comprising abacavir and lamivudine
InactiveUS20050171127A1High drug loadingMaintain good propertiesBiocideAntiviralsCyclopenteneImmunodeficiency virus
A pharmaceutical composition comprising (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol and (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one, in an amount which achieves antiviral efficacy, a process for the preparation of such a composition, and a method of inhibiting human immunodeficiency virus (HIV) which comprises administering such a composition to an HIV infected patient is disclosed.
Owner:SMITHKLINE BECKMAN CORP
Anti-AIDS traditional Chinese medicine compound composite and preparation method thereof
ActiveCN101417049AImprove immunityEliminate symptomsAntiviralsUnknown materialsSide effectRaw material
The invention relates to a compound composition of a traditional Chinese medicine, in particular to an anti-AIDS compound composition and a preparation method thereof. Pilos antler, wolfberry fruits, herba epimedii, rhizoma curculiginis, herba cistanches and other traditional Chinese raw materials are extracted, concentrated, decocted, filtered, concentrated, dried in vacuum, smashed and mixed to prepare the anti-AIDS compound composition. The compound composition is mainly used for treating AIDS patients and HIV-infected patients, and can restrain and kill AIDS virus improve the immunity of human bodies and increase the number of CD4 cells in human body, thereby eliminating all symptoms of patients and restoring the strength and weight of patients so that the patients can work and live healthily. The anti-AIDS compound composition is efficient and safe, causes no drug resistance and has no toxic side effects, no rebound after drug withdraw and wide application range. In addition, the anti-AIDS compound composition can be applied to children and pregnant women as well as all varieties and stages of HIV-infected patients.
Owner:北京同馨堂中医药科技发展有限公司
Combinations Of A Pyrimidine Containing Nnrti With Rt Inhibitors
InactiveUS20080200435A1High resistance barrierTakes longBiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorNucleotide
The present invention concerns combinations of a pyrimidine containing NNRTI with nucleoside reverse transcriptase inhibitors and / or nucleotide reverse transcriptase inhibitors useful for the treatment of HIV infected patients or for the prevention of HIV transmission or infection.
Owner:TIBOTEC NV +1
Composition and methods used during Anti-hiv treatment
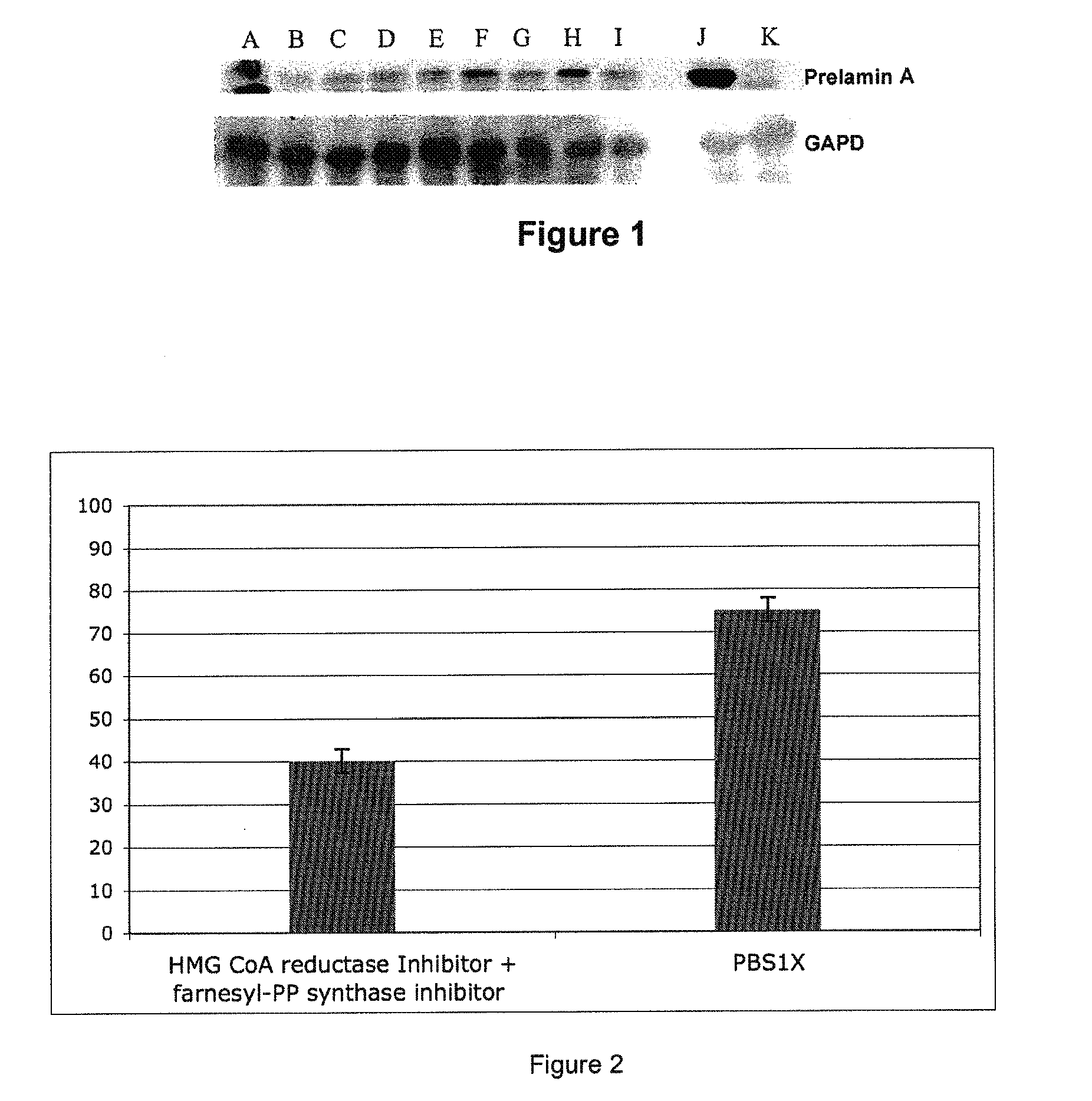
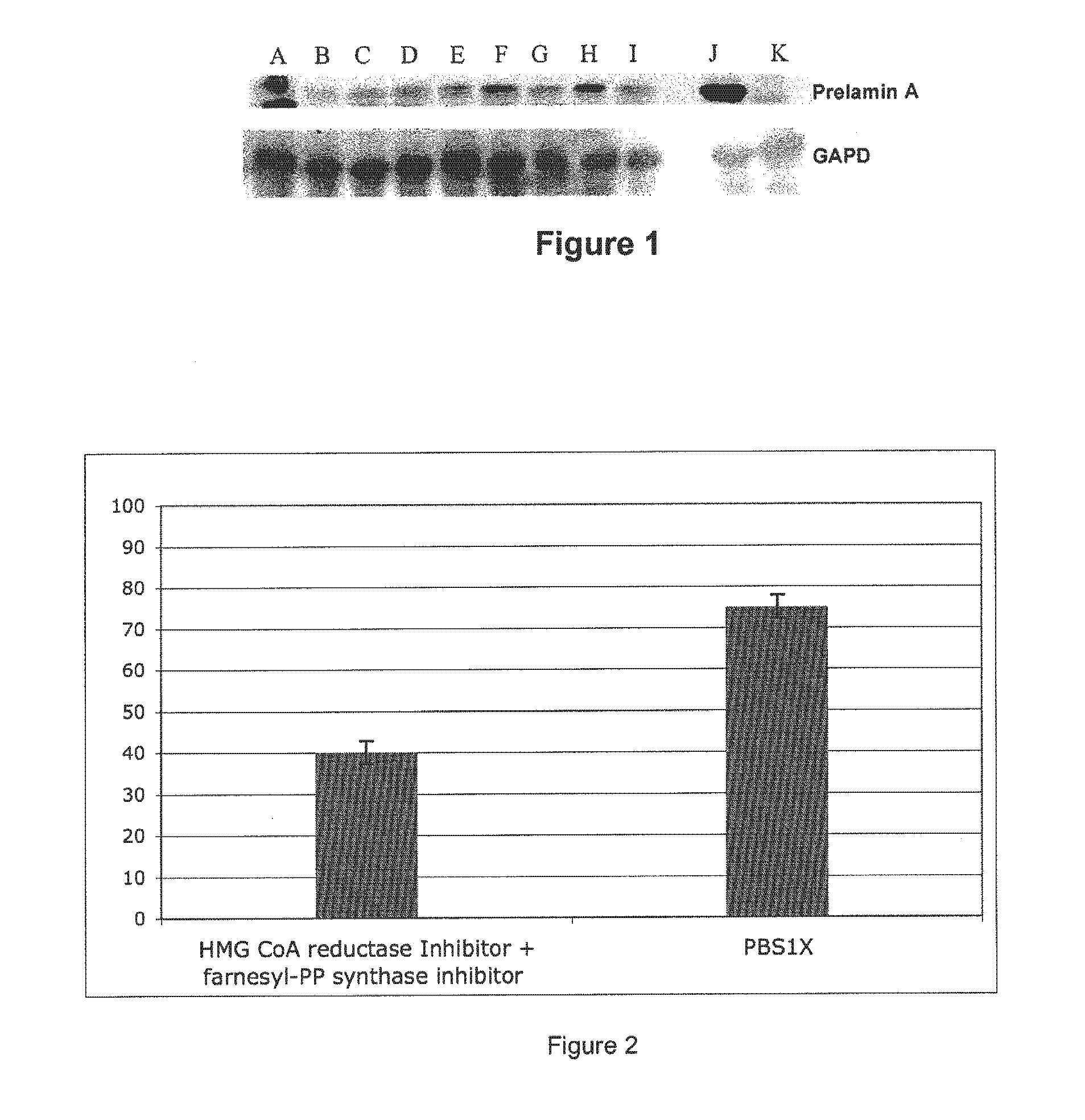
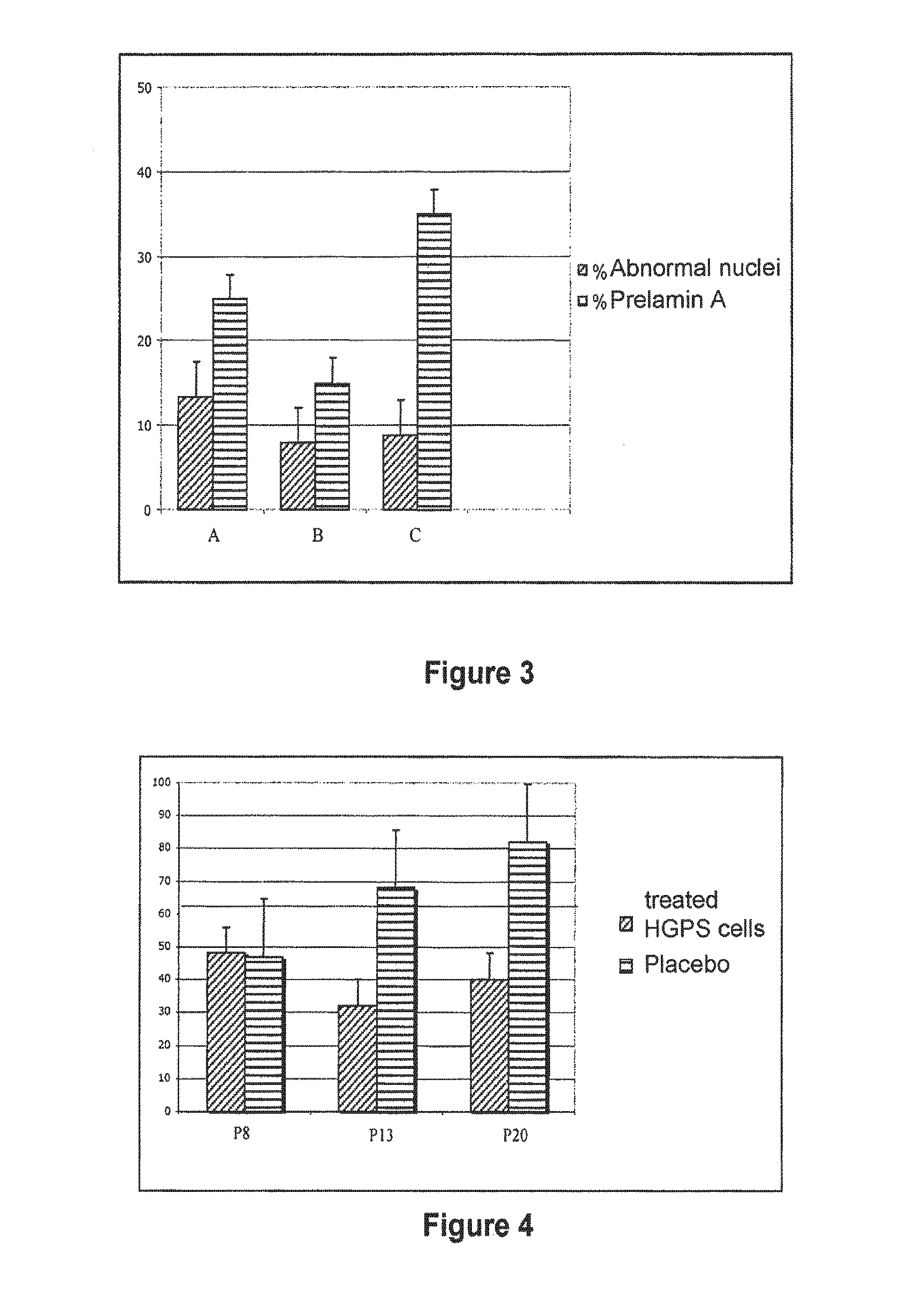
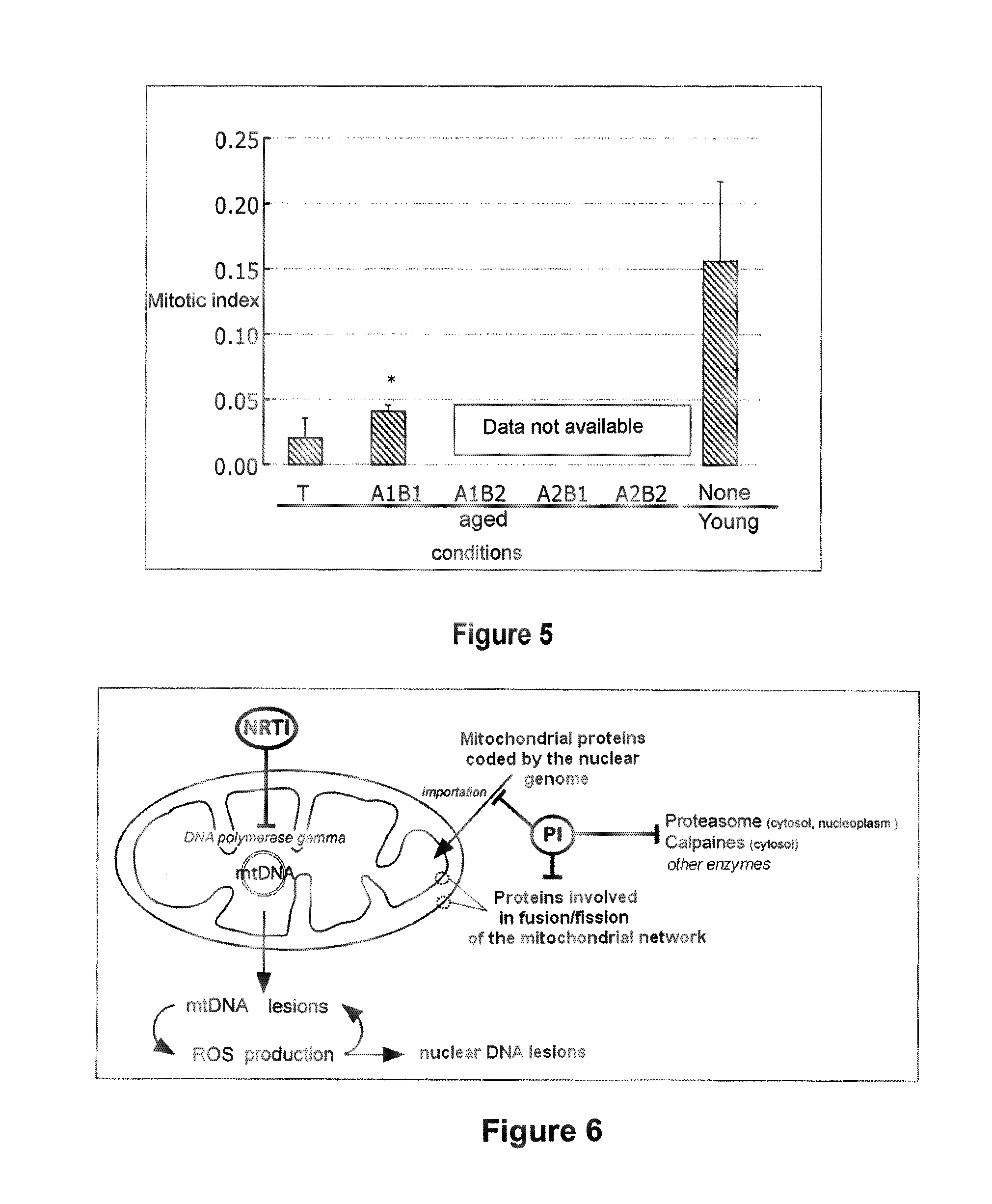
InactiveUS20110046091A1Restoration of the normal phenotypeSymptoms improvedBiocideMetabolism disorderPremature agingSide effect
This invention relates to a composition comprising an anti-HIV treatment and a treatment for side effects of said anti-HIV treatment in an HIV-infected patient. This invention is, for example, very useful in the treatment of side effects caused by certain anti-HIV treatments, for example premature aging and lipodystrophy, which can be caused by protease inhibitors or reverse transcriptase inhibitors. The composition of this invention includes at least one hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, at least one farnesyl-pyrophosphate synthase inhibitor, and at least one anti-HIV agent. One of the processes for treating an HIV-infected patient includes, in any order, the following steps: (i) administration of a mixture including at least one hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor and at least one farnesyl-pyrophosphate synthase inhibitor and (ii) administration of an anti-HIV agent, in which the administrations are concomitant, successive or alternative.
Owner:UNIV DAIX MARSEILLE
Use of T cell comprising chimeric antigen receptor (CAR) modification for preparing cell drug
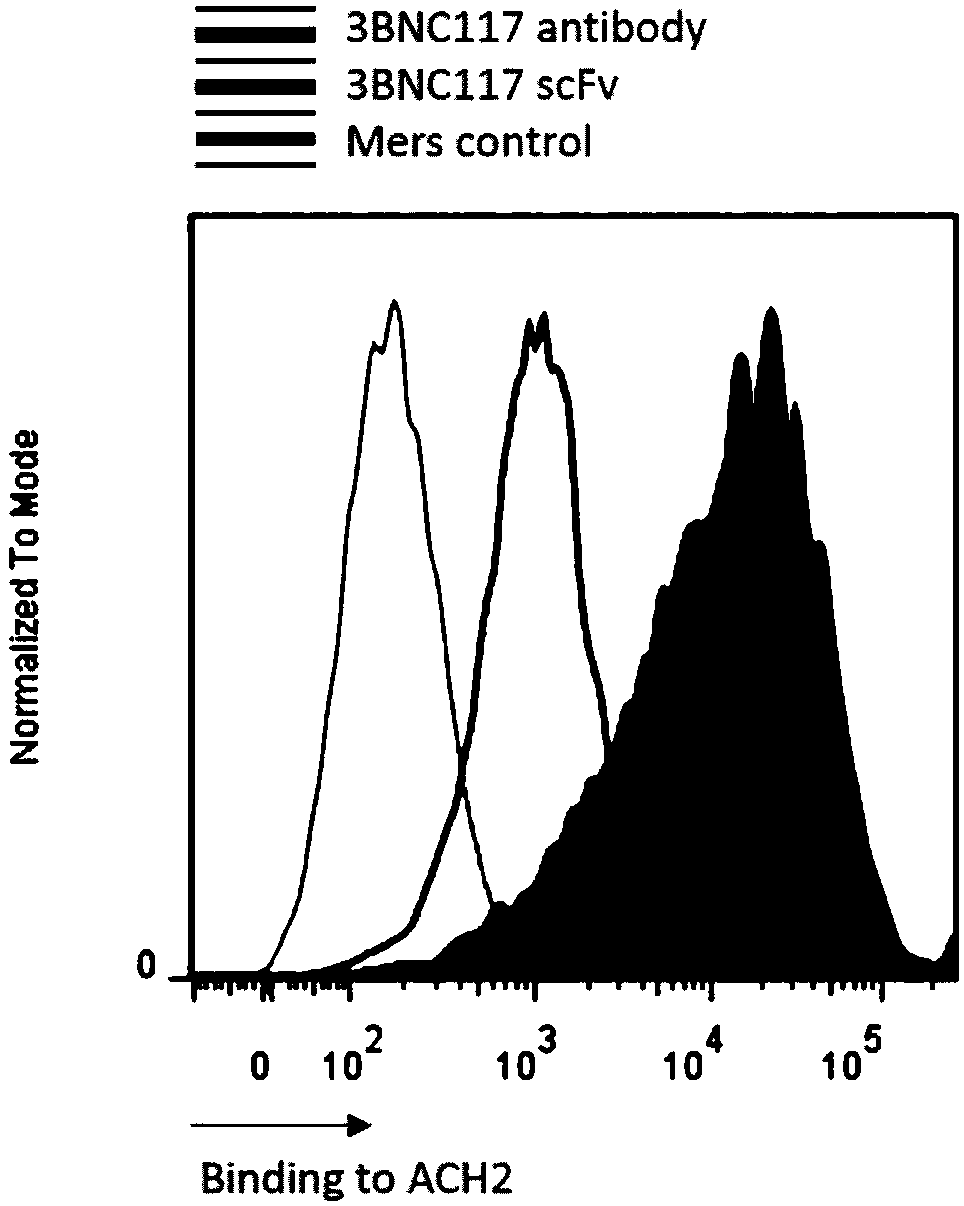
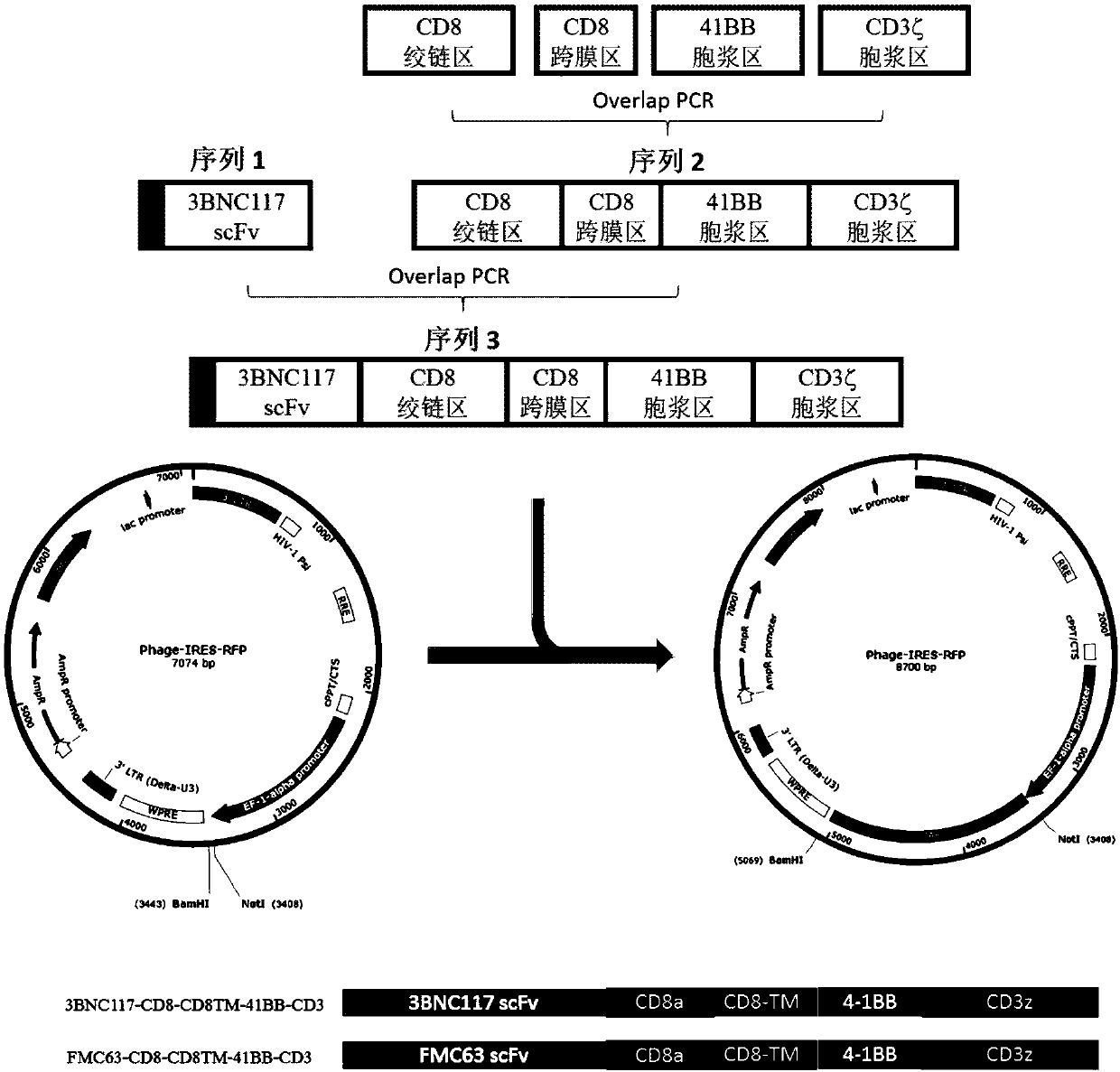
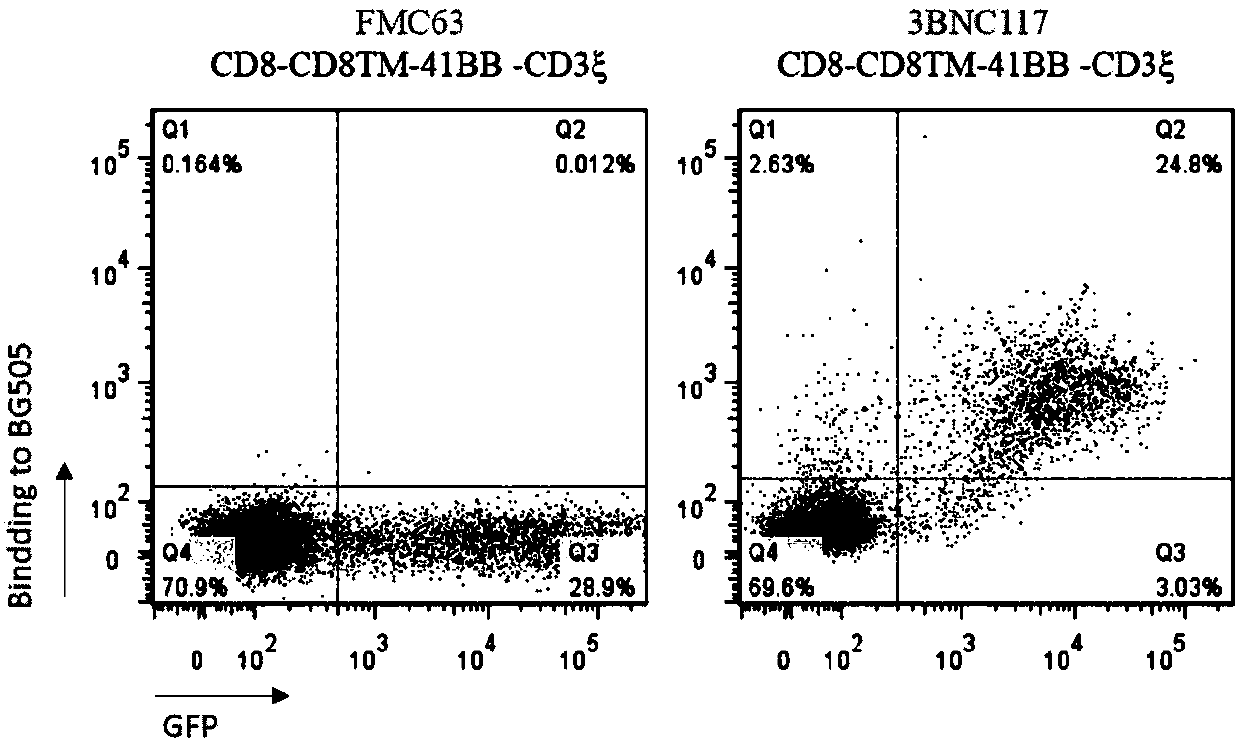
The invention discloses a T cell comprising a chimeric antigen receptor (CAR) modification, a preparation or a medicine prepared from the T cells, the use of the T cells for preparing a cell drug, andthe preparation or the drug for treating and removing a cell storage bank of human immunodeficiency virus (HIV) in a HIV infected patient. The invention further discloses a cell immunotherapy using gp120 antibody CART, and specifically using 3BNC117 scFv-CART, which achieves MHC independent recognition and killing specific for HIV latent cells, so that the goal of eradicating AIDS is achieved.
Owner:TSINGHUA UNIV +1
Novel herbal formulation for the modulation of immune system of HIV infected patients and a process of preparation thereof
InactiveUS20130071435A1Enhance body resistanceImprove body immunityNervous disorderAntipyreticHerbal preparationsChromatographic separation
An herbal formulation for the modulation of immune system of HIV infected patients and a process of preparation thereof are provided. The process includes (i) preparing a hydromethanolic extract of at least one plant selected from Hippophae rhamnoides, Convolvulus pluricaulis, Withania somnifera, Ocimum sanctum, and Cynodon dactylon at 80-90° C., maintaining the pH of the solution between 6-7; (ii) separating the active compound chromatographically; and (iii) subjecting the active compounds to the step of molecular characterization.
Owner:SUNIL KUMAR SWAMY RAJAN & ARUN KUMAR SWAMY RAJAN
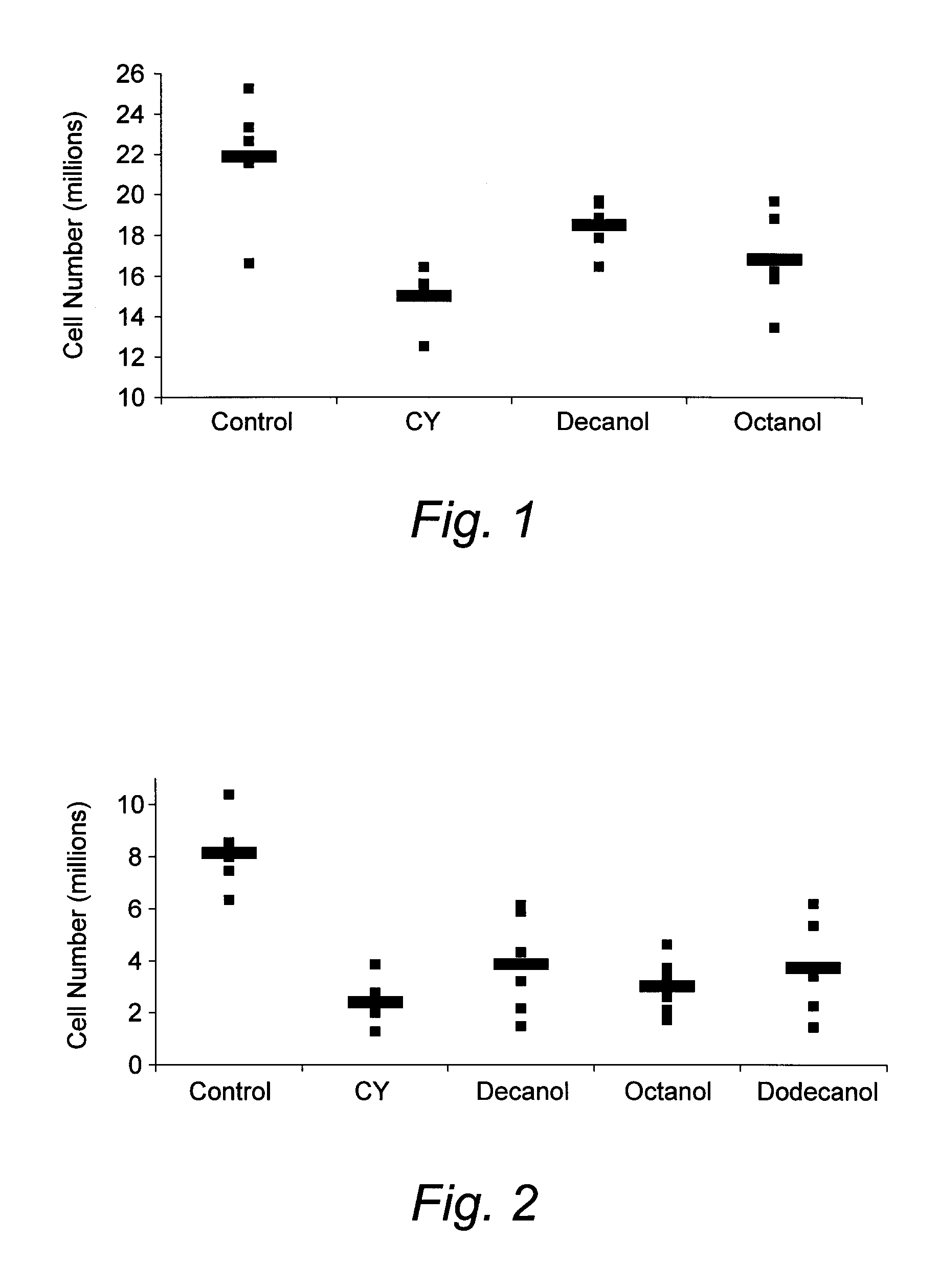
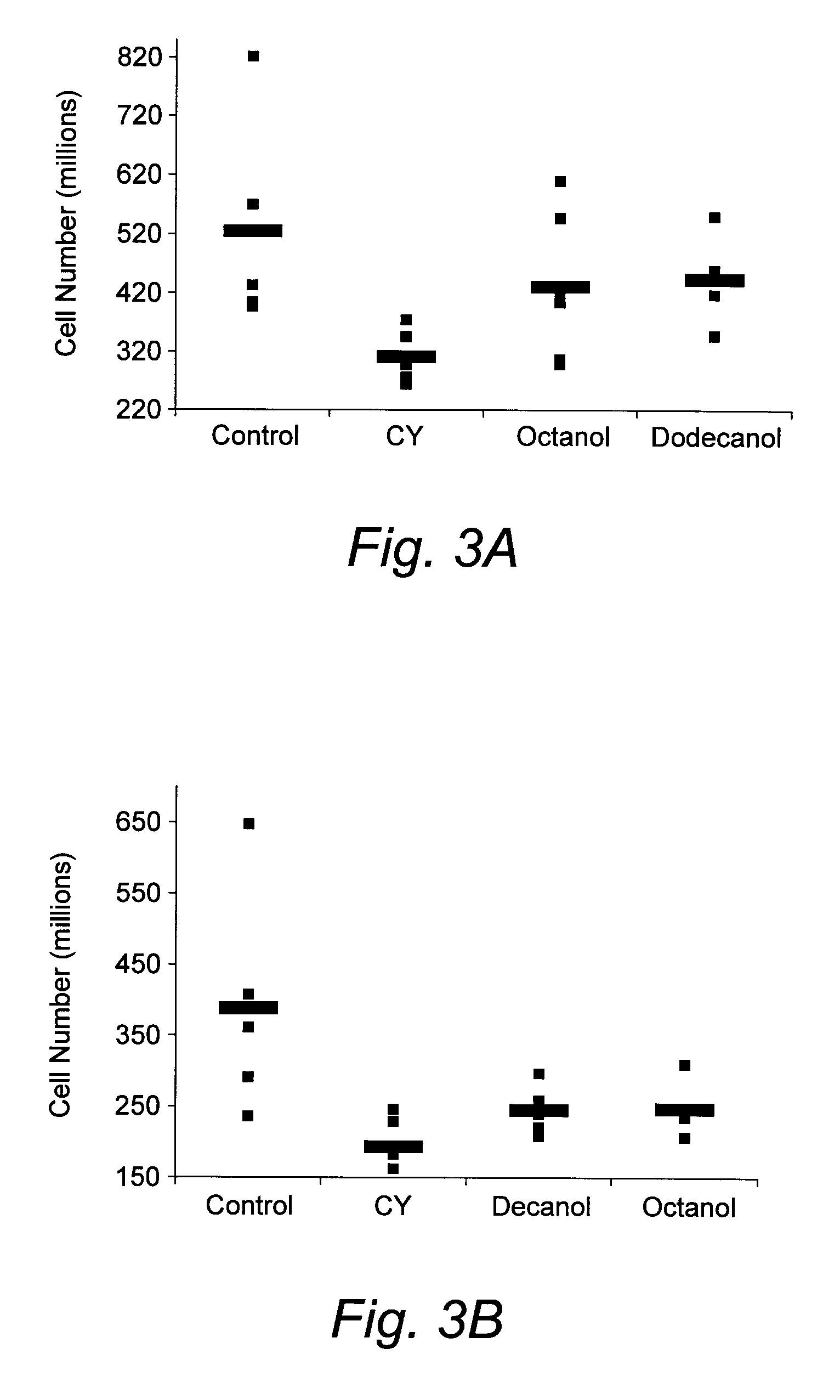
Medium-Chain Length Fatty Alcohols as Stimulators of Hematopoiesis
InactiveUS20080051324A1Good curative effectReducing and eliminating chemotherapyBiocideHydroxy compound active ingredientsProgenitorNutritional deficiency
Owner:PROMETIC PHARMA SMT LTD
CCR5 antagonist for enhancing immune reconstitution and treating opportunistic infection in HIV patients
InactiveCN101466376AImprove immune reconstitutionPrevent opportunistic symptomsAntibacterial agentsOrganic active ingredientsCXCR4HIV receptor
The present invention relates to the use of a CCR5 antagonist in an HIV infected patient to enhance their immune reconstitution and so treat to HIV related opportunistic conditions resulting from the immunocompromised state of the HIV patient. The invention also allows treatment with a CCR5 antagonist of patients having a CXCR4 using viral population since such patients will also benefit from an increase in their CD4 and / or CD8 cell count.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
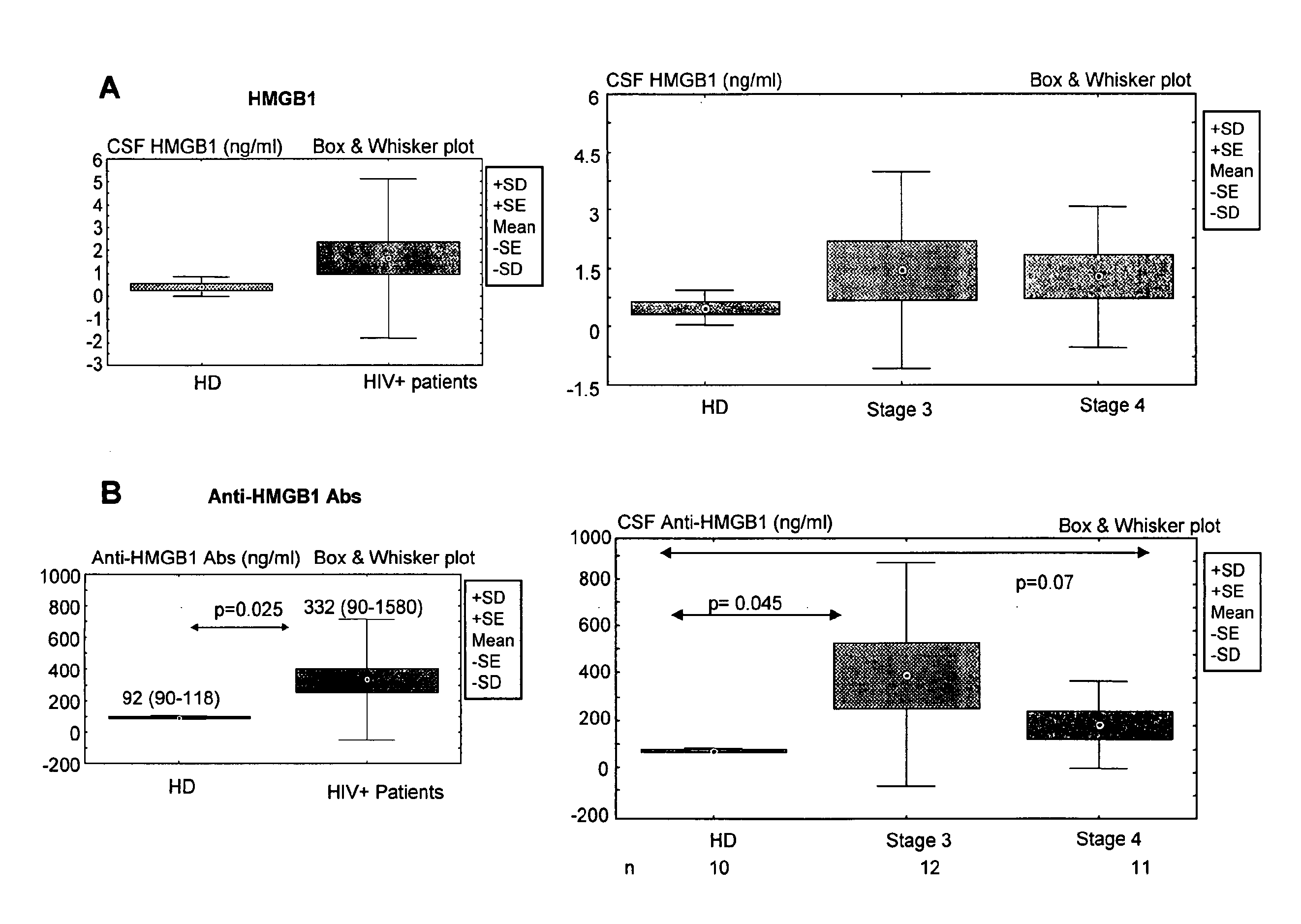
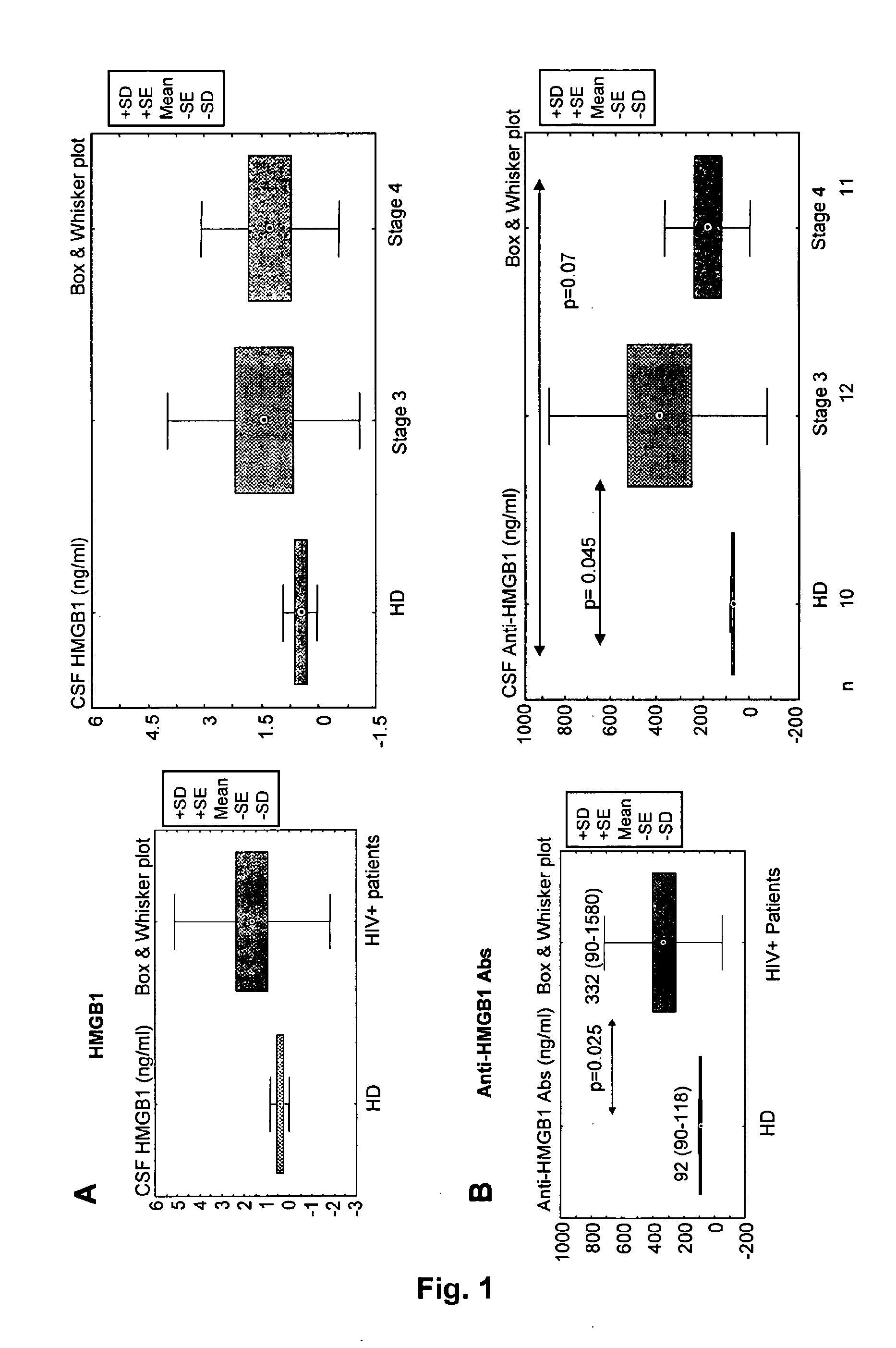
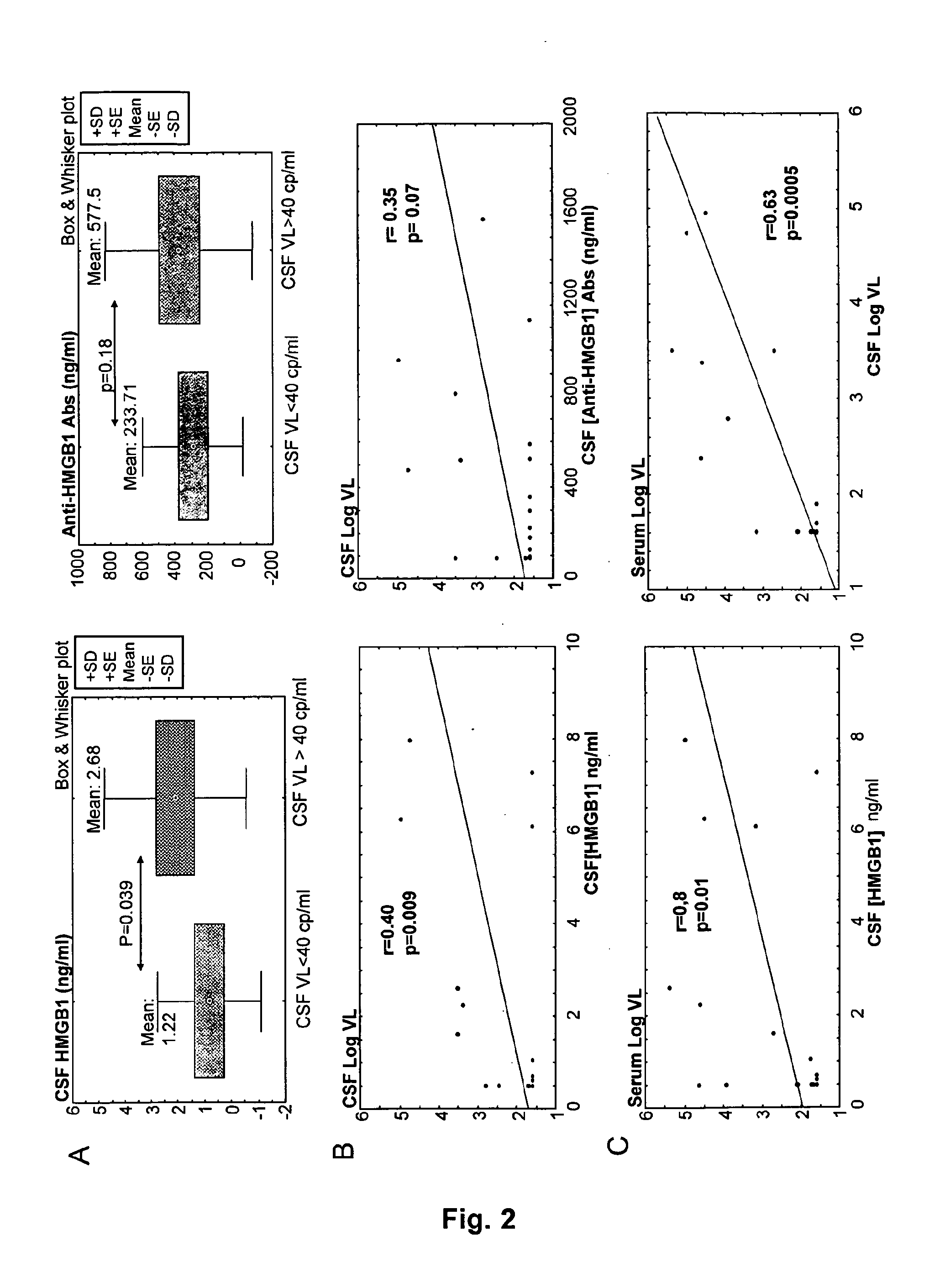
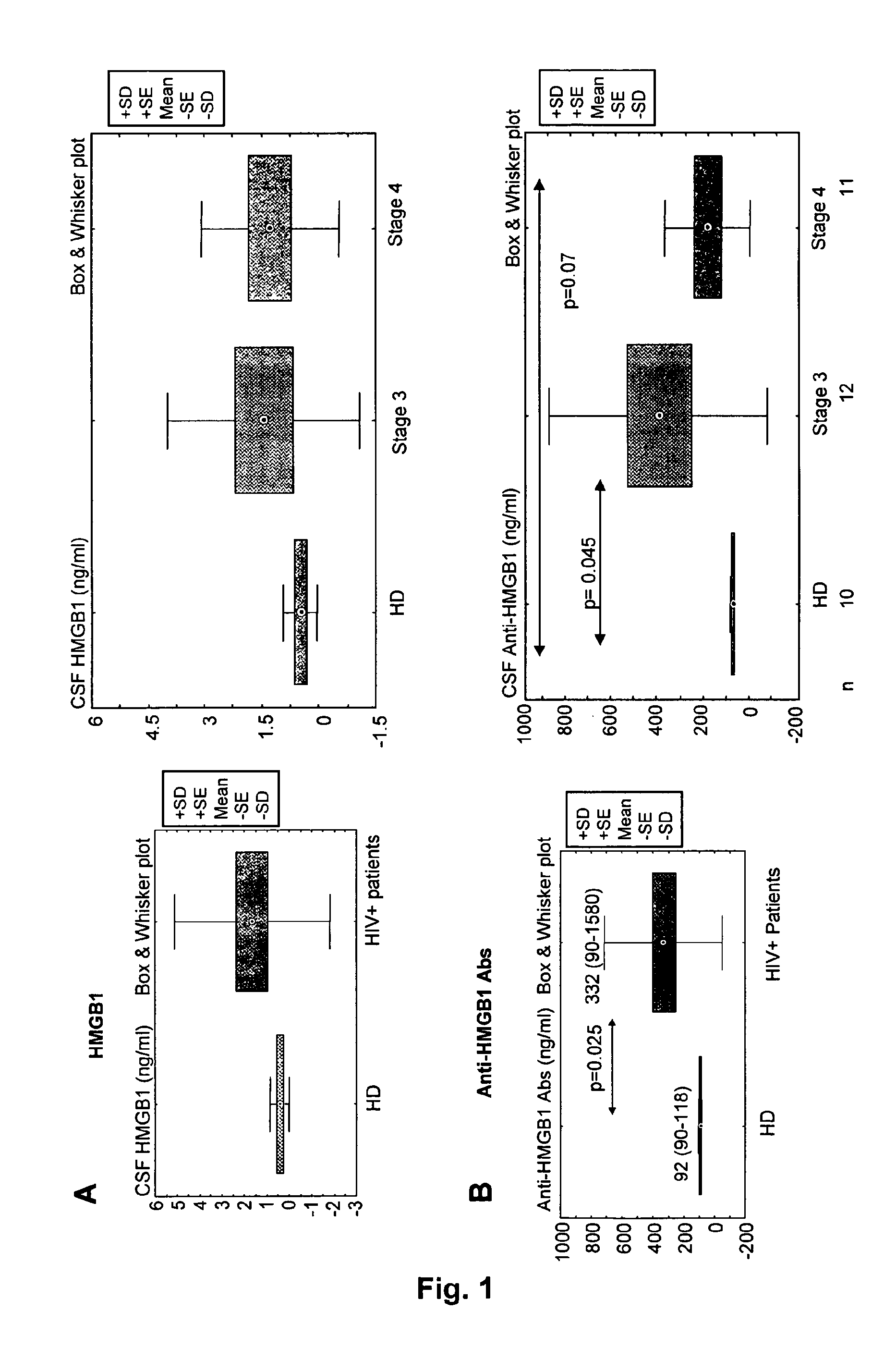
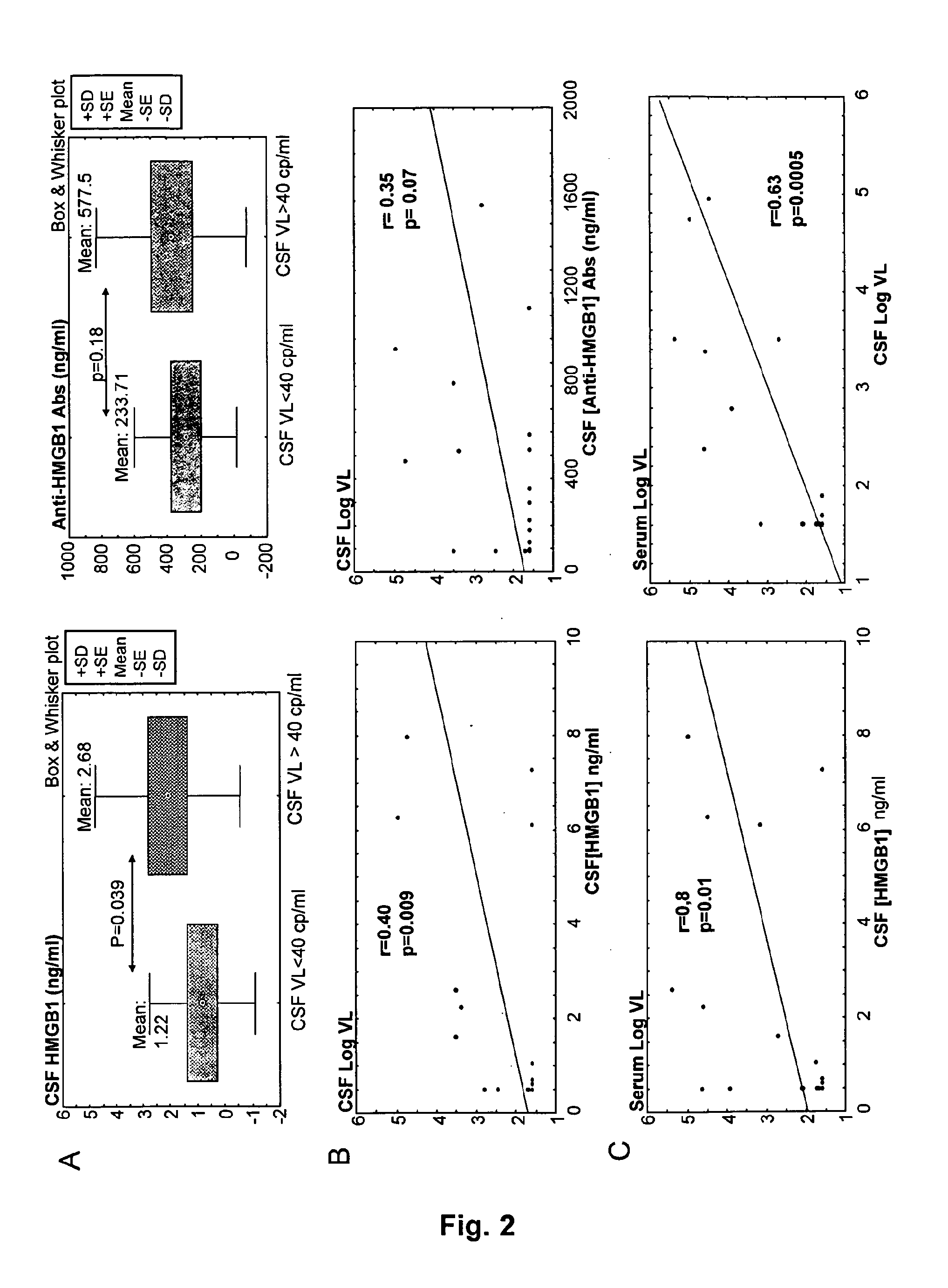
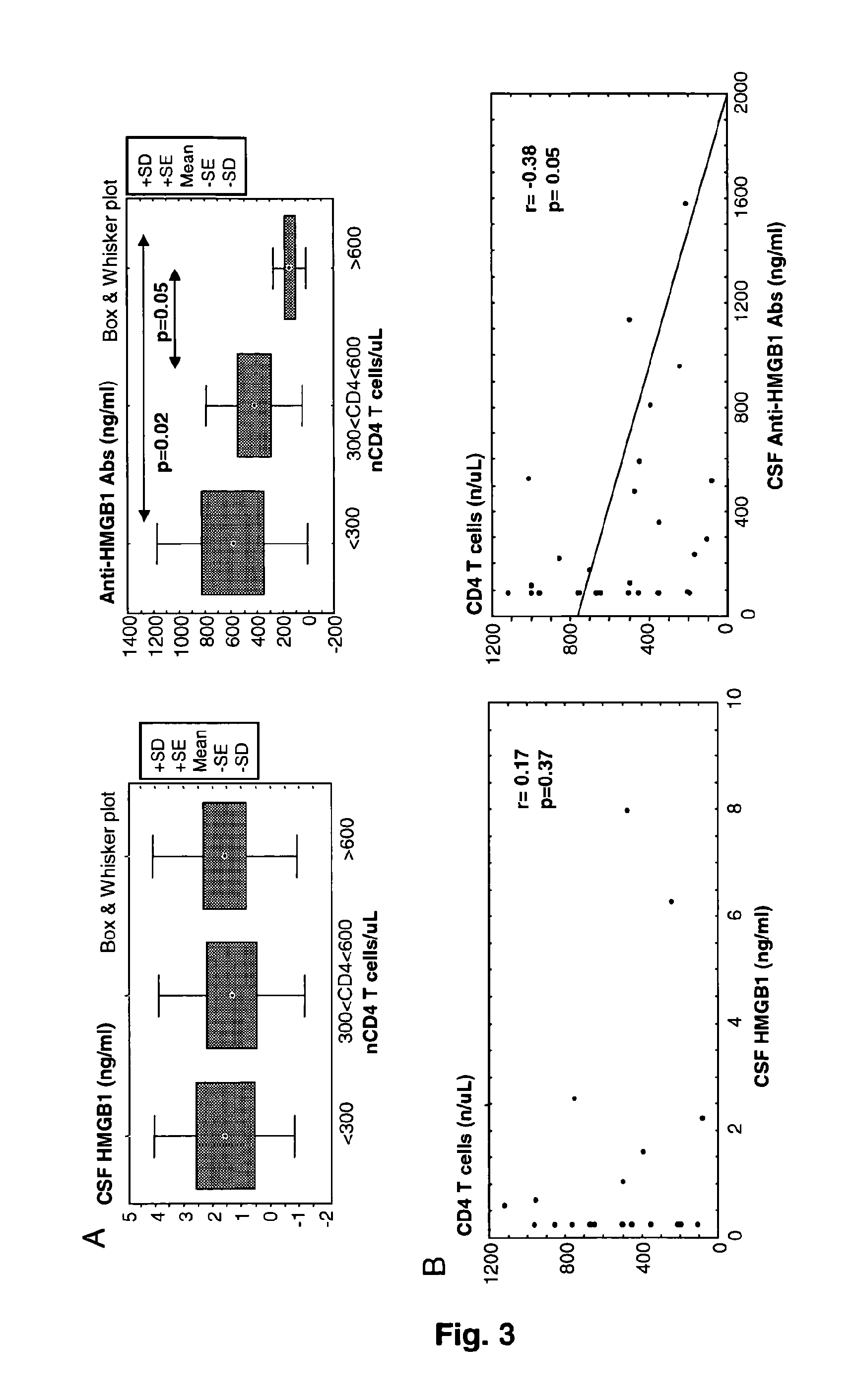
Hmgb1 and Anti-hmgb1 antibodies for the prognostic of neurological disorders
ActiveUS20130065221A1Microbiological testing/measurementVaccination/ovulation diagnosticsDiseaseNervous system
The invention relates to in vitro method for quantitating the antibodies specific for High mobility group box I (HMGB1) contained in a sample, in particular a serum sample or a cerebrospinal fluid sample obtained from a patient, and the use of this method in the prognostic and / or diagnosis of neurological disorders. These methods are in particular applicable to the monitoring of the human immunodeficiency virus (HIV) infection of a subject who is known to be infected with HIV and in the prognostic and / or diagnostic of the state of progression of Acquired immune deficiency syndrome (AIDS) or the state of progression toward AIDS, in particular the state of progression or the state of progression toward neurological disorders associated with AIDS. Finally, the invention is also about method to determine the immune deficiency or level of immune activation of a patient, in particular a HIV-infected patient.
Owner:INST PASTEUR
Glutathione reductase for therapy and prophylaxis of aids
InactiveUS7045323B2Peptide/protein ingredientsPharmaceutical delivery mechanismMedicineGlutathione reductase
The present invention concerns the use of glutathione-reductase (GSSG reductase) for the preparation of a medicament for the treatment of HIV-infected patients, seropositive or already affected by AIDS, both for prophylactic and therapeutic use.
Owner:ANSOVINI RAFFAELE
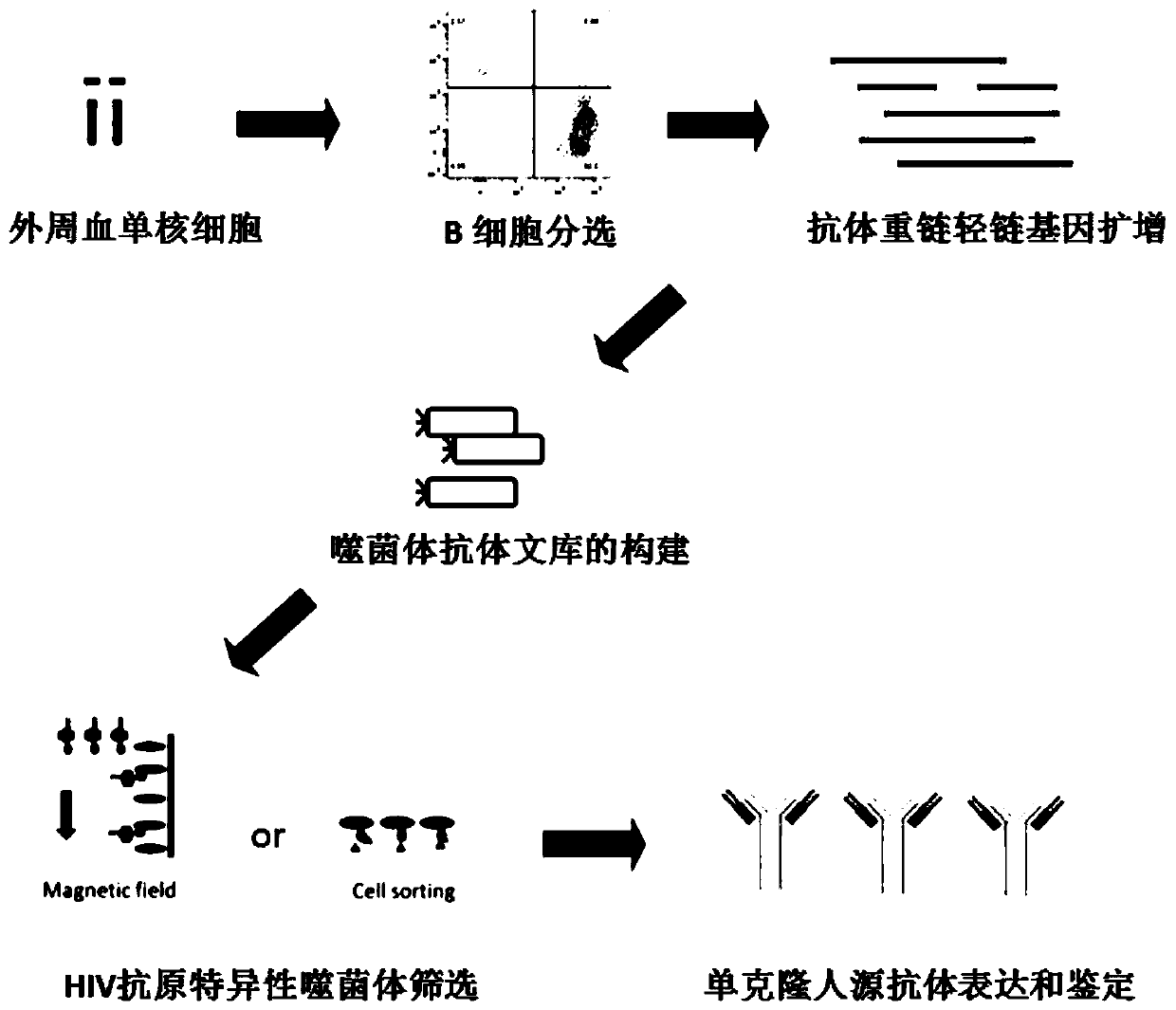
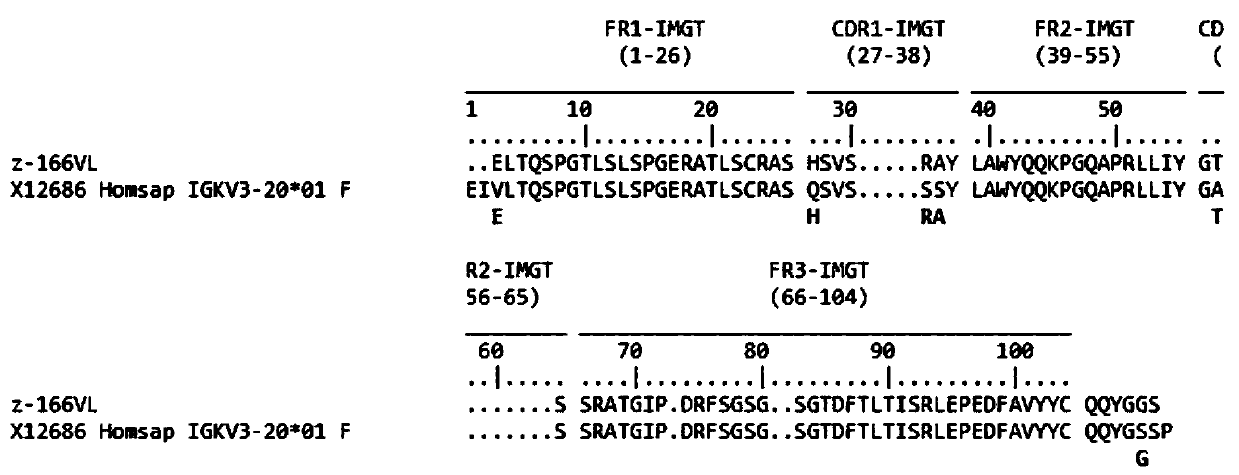
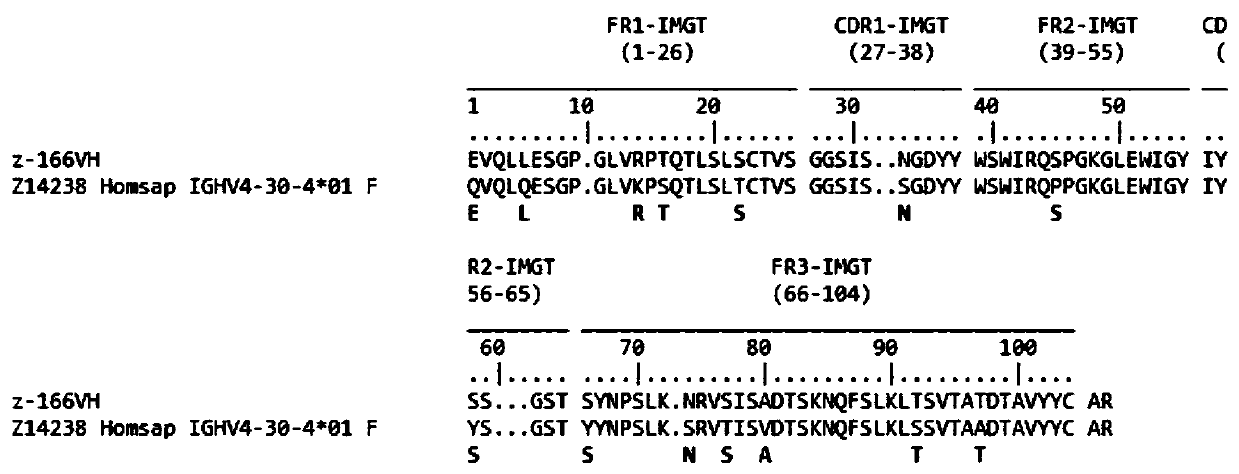
Human anti-HIV gp 120 specific antibody Z166 and application method thereof
The present invention discloses to a human anti-HIV gp 120 specific antibody Z166, provides amino acid sequences of the antibody and an active fragment thereof, provides coding genes of the antibody or the active fragment thereof, and simultaneously provides application methods of the antibody and the active fragment thereof in preparing medicines for treating or preventing HIV infection diseasesand application methods in preparing HIV diagnosis or detection reagents. The antibody Z166 can effectively neutralize a plurality of HIV virus subtypes, is used for treating HIV infected patients, effectively mediates killing of effector cells to HIV infected cells, can also mediate the killing (antibody-dependent cell-medicated cytotoxicity, ADCC) of the effector cells to an HIV envelope proteinstable expression cell line TF 228, and keeps a killing percentage of 30% or above when antibody concentration is 0.2 [mu]g / ml or more. The provided antibody Z166 can be used for treating the HIV infected patients and is suitable for antibody medicines based on all treatments of diseases caused by HIV based on the Z166.
Owner:GUIZHOU MEDICAL UNIV
HMGB1 and anti-HMGB1 antibodies for the prognostic of neurological disorders
ActiveUS8728748B2Snake antigen ingredientsVaccination/ovulation diagnosticsNervous systemImmunodeficiency
The invention relates to in vitro method for quantitating the antibodies specific for High mobility group box I (HMGB1) contained in a sample, in particular a serum sample or a cerebrospinal fluid sample obtained from a patient, and the use of this method in the prognostic and / or diagnosis of neurological disorders. These methods are in particular applicable to the monitoring of the human immunodeficiency virus (HIV) infection of a subject who is known to be infected with HIV and in the prognostic and / or diagnostic of the state of progression of Acquired immune deficiency syndrome (AIDS) or the state of progression toward AIDS, in particular the state of progression or the state of progression toward neurological disorders associated with AIDS. Finally, the invention is also about method to determine the immune deficiency or level of immune activation of a patient, in particular a HIV-infected patient.
Owner:INST PASTEUR
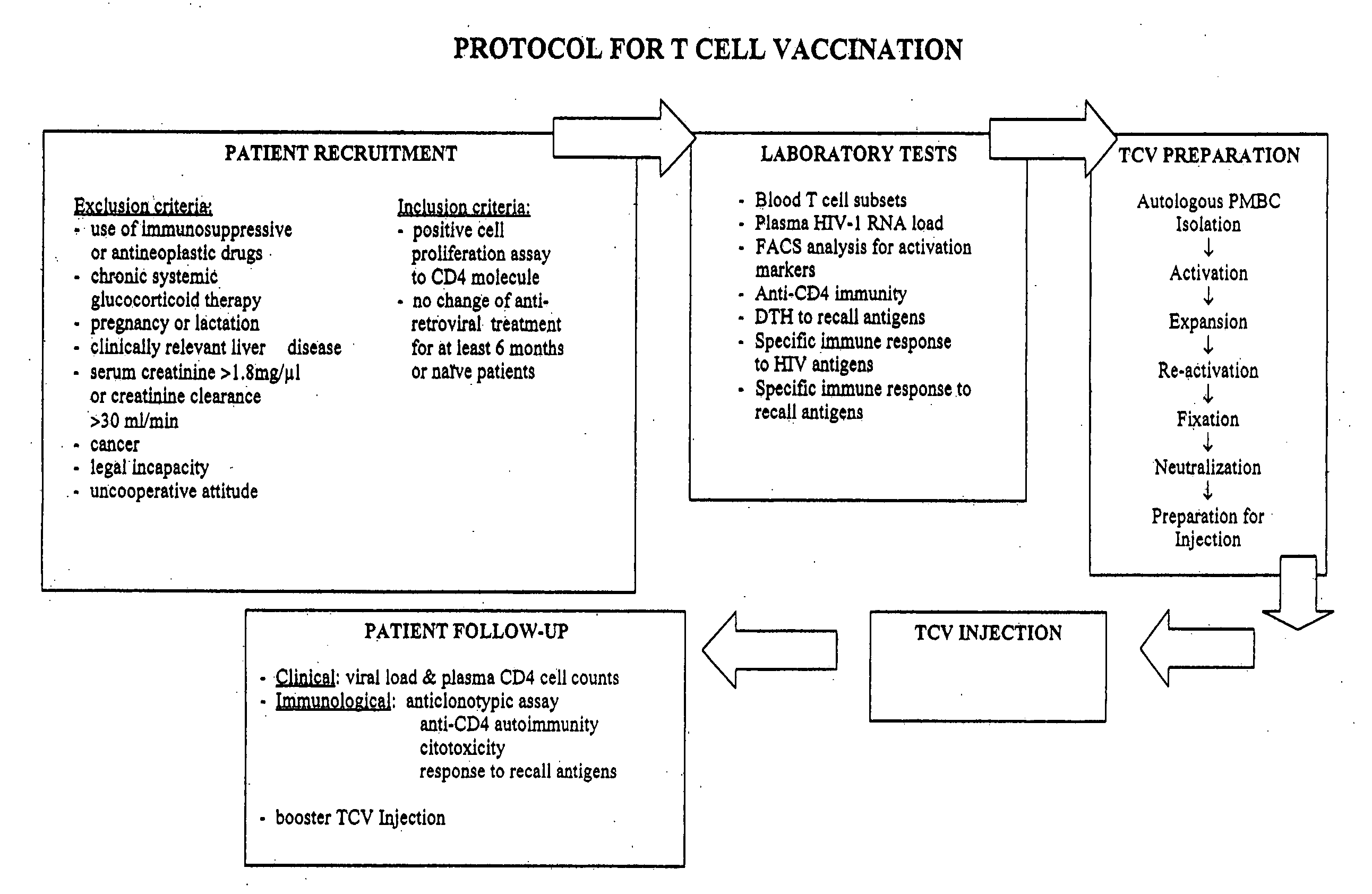
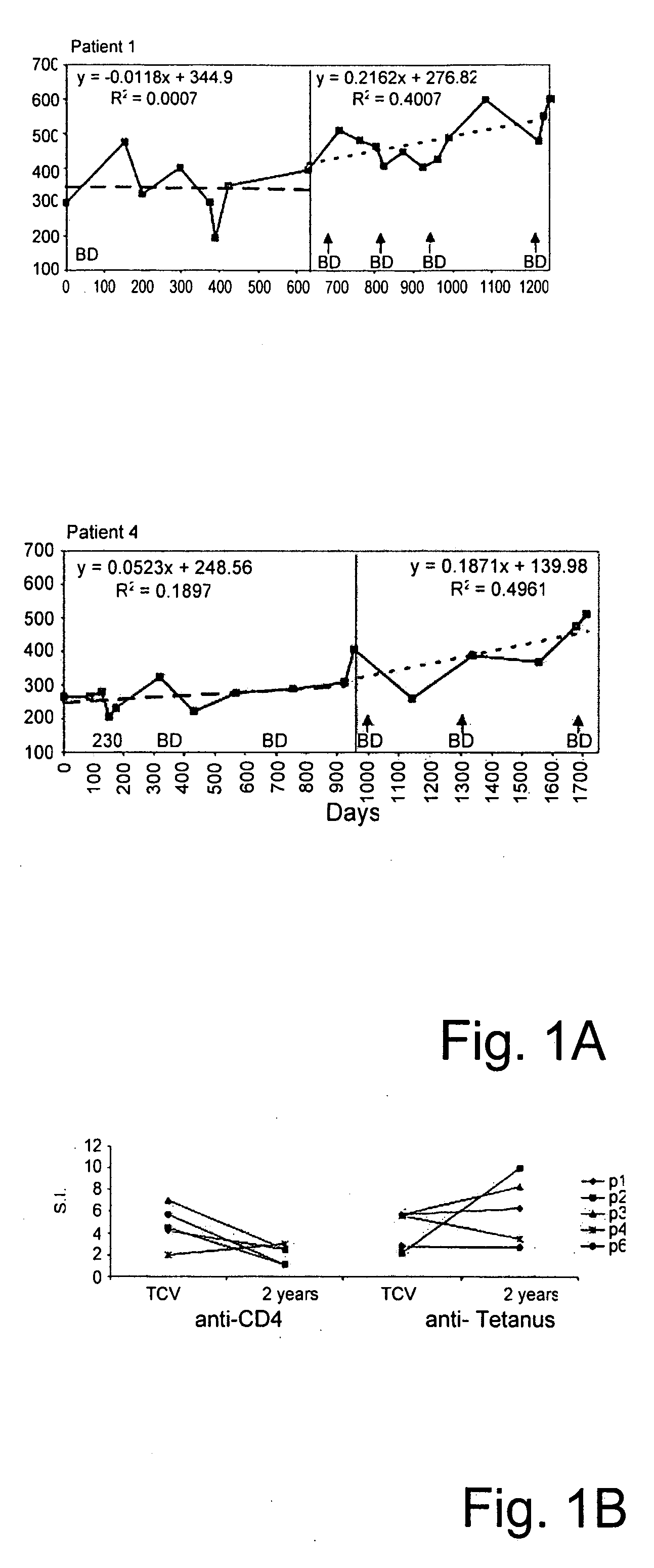
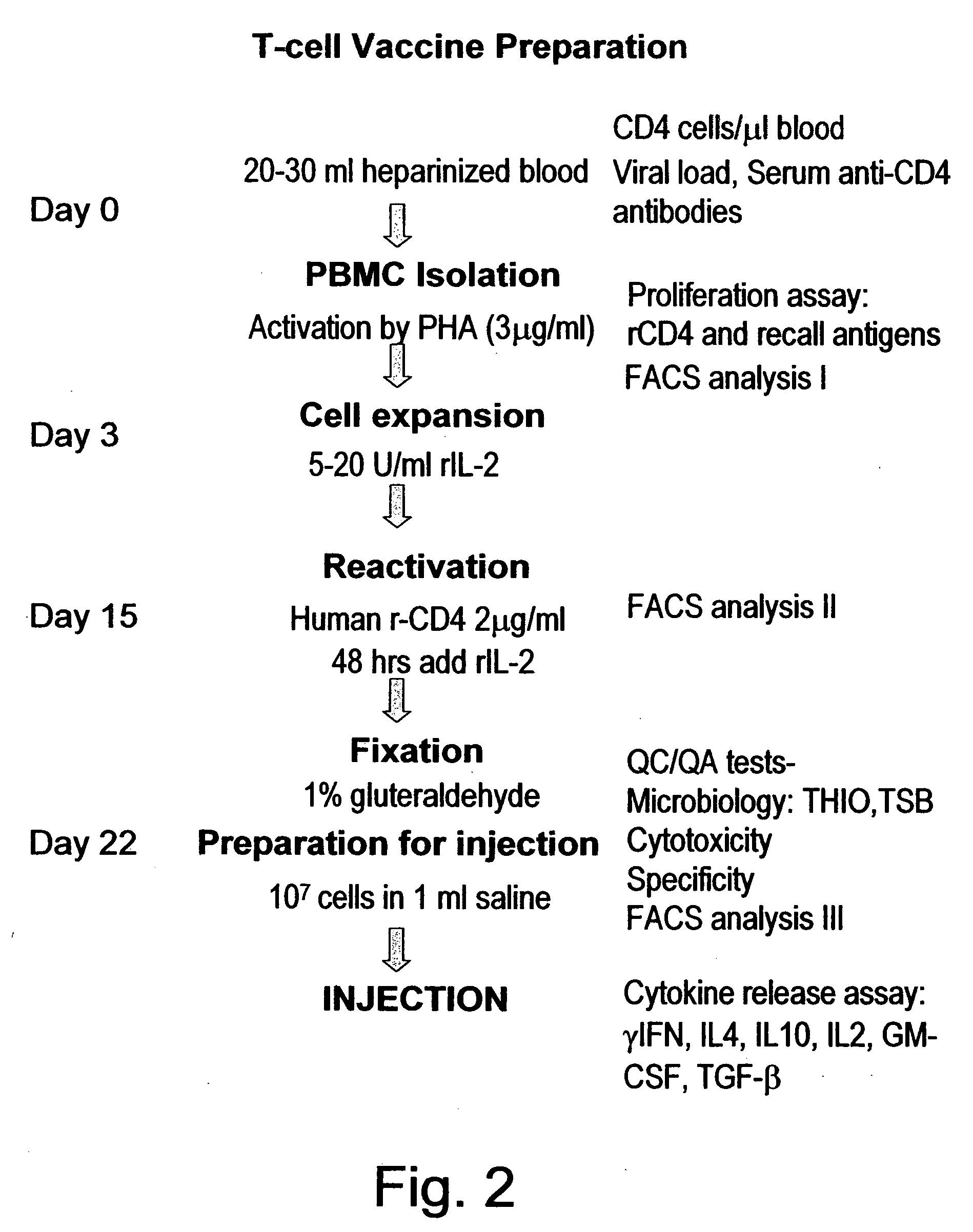
T-cell vaccination in the treatment of HIV infection
InactiveUS20070122877A1Microbiological testing/measurementSnake antigen ingredientsImmunodeficiencyCD4 antigen
A method for the preparation of a T cell vaccine for the treatment of immunodeficient HIV-infected patients is described herein, based on the enrichment of autologous CD4-reactive CD8 T cells. Also described is a protocol for the implementation of T cell vaccination in immunodeficient HIV-infected, as well as a method of treatment, based on the T cell vaccine developed herein. Finally, kits for preparing the T cell vaccine as well as for implementing the protocol are also provided.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
DNA-based plasmid formulations and vaccines and prophylactics containing the same
The invention is a general method for improving the performance of the DNA-based vaccines. The method utilizes a complex DNA-generated profile of antigens to extend the effects of DNA-based vaccines and to broaden the immune response. This broadened immune response in turn improves the protection of the recipient from divergent (but related) strains of a pathogen. In addition, it effectively improves the efficacy of DNA-based vaccines used for treatment of viral diseases, including acquired immunity disorder (AIDS). One embodiment, where the target viral pathogen is HIV (the causative agent for aids), the method identifies an orderly set of plasmids of related sequences that may be used to prime a broad and strong immune response to HLA-restricted viral antigens. This mixture of plasmids is thus capable of priming an appropriate immune response to reduce the viral burden in HIV infected patients or to protect uninfected patients from HIV infection.
Owner:LASHER ALFRED W +2
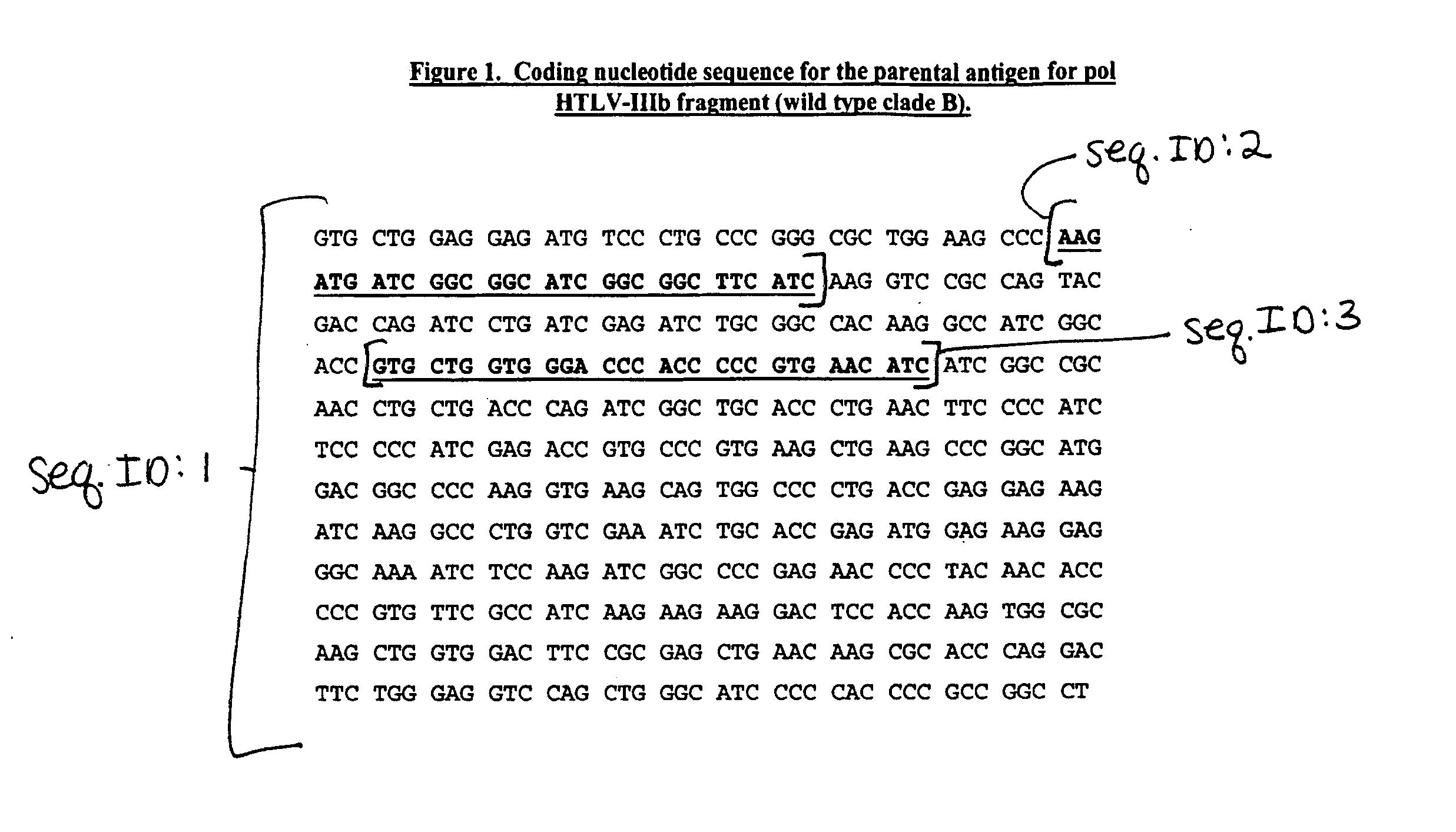
Primer composition for identifying HIV (human immunodeficiency virus) in assistance mode and application thereof
ActiveCN102250890AMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceImmunodeficiency virus
The invention discloses a primer composition for identifying HIV (human immunodeficiency virus) in assistance mode and application thereof. The primer composition provided by the invention is composed of a DNA represented by a sequence 1 in a sequence table, a DNA represented by a sequence 2 in the sequence table, a DNA represented by a sequence 3 in the sequence table, and a DNA represented by a sequence 4 in the sequence table; the primer composition can be used for (a) identifying the human immunodeficiency virus (HIV) in assistance mode, or (b) identifying the HIV-infected patient in assistance mode. The primer composition has important application value in identification of HIV and diagnosis of HIV-infected patients in assistance mode, and has great influence on social health.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
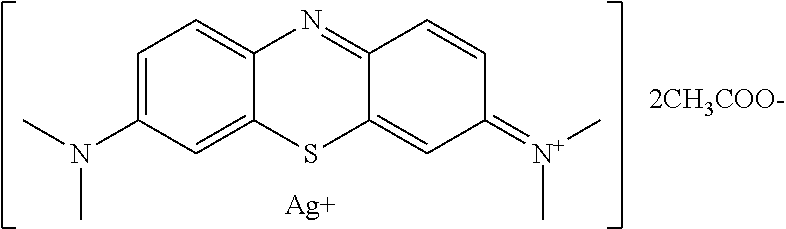
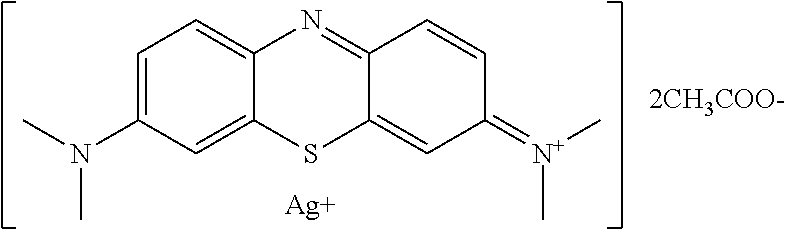
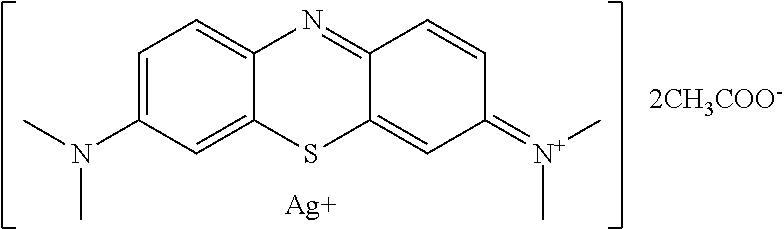
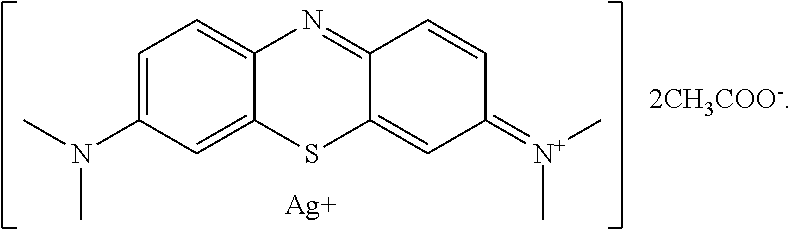
Antiviral Water-Soluble Product with Anti-HIV Effect, Based on Ionic Silver and Methylene Blue Compound; Method of Its Production and Examples of HIV-infected Patients' Treatment
A new antiviral water-soluble pharmacological product with anti-HIV effect, is based on ionic silver and methylene blue compound. The new substance is produced in the form of water-soluble powder that can be used for the preparation of finished dosage forms for treating viral infections, especially HIV infection. The product has a potential ability to radically suppress the infectious agents without leading to any mutations and resistance of viruses. The composition of the new product proposed under the conventional name Argothiazin-A™ corresponds to the chemical formula: C20H24AgN3O4S.
Owner:SVETLIK HARVEY E +5
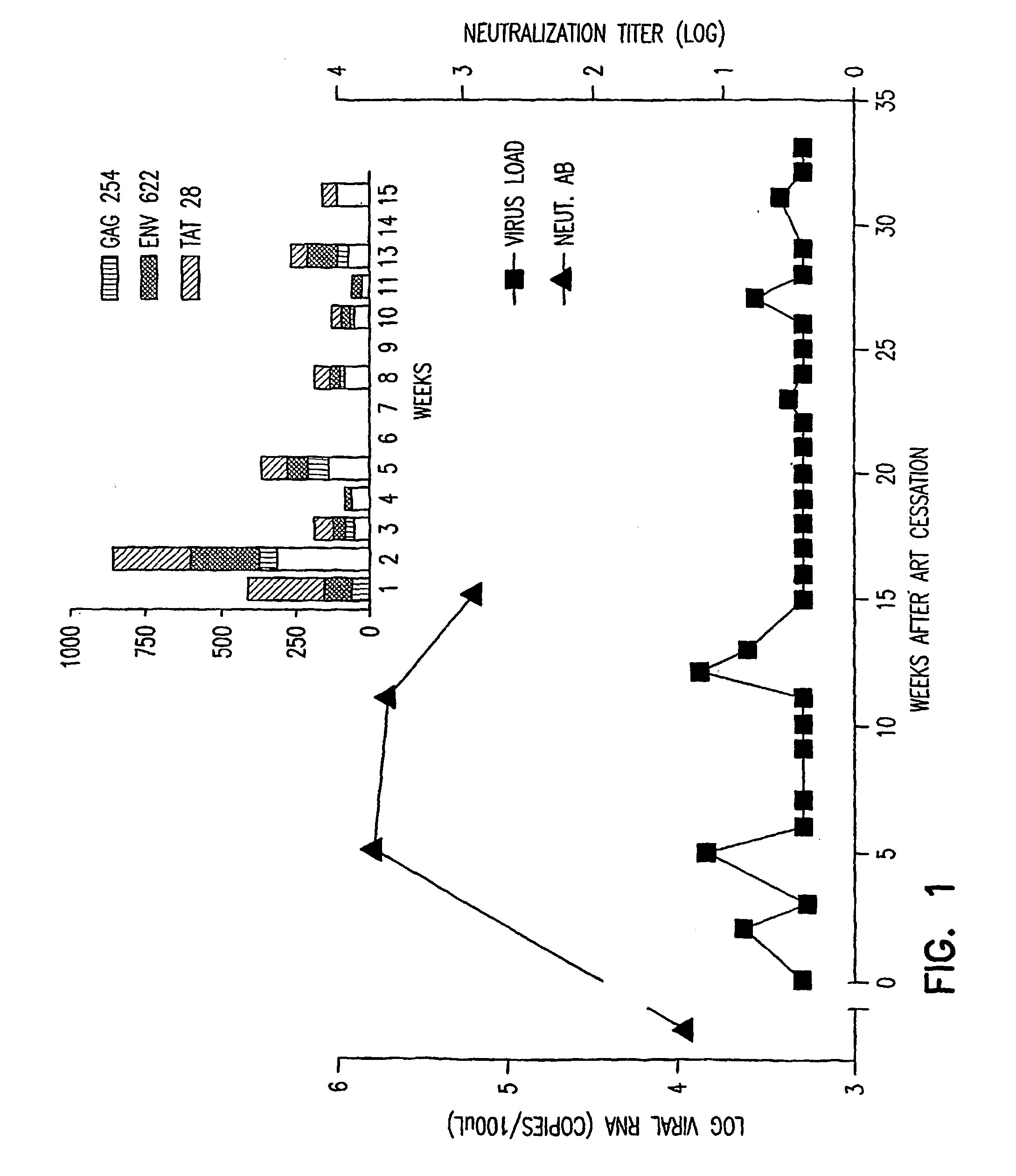
Immunotherapy regimens in hiv-infected patients
InactiveUS20060094006A1Good curative effectImprove satisfactionViral antigen ingredientsMicrobiological testing/measurementImproved methodImmunotherapy
This invention relates to an improved method of maintaining an immuno-protective response in persons infected with a retrovirus during early infection or after highly active anti-retroviral therapy.
Owner:SANOFI PASTEUR LTD +1
Method for evaluating curative effect of medication to cure HIV through detecting change of TCR gene of human T cell receptor before and after treatment
InactiveCN1480537AReduce demandConsistent positioningMicrobiological testing/measurementAfter treatmentCurative effect
A method for evaluating the curative effect of the medicine to treat HIV infection features that a particular primer is designed to complete PCR in order to amplify the variation of T cell receptor (TCR) gene of HIV infected patient before and after the medicine is applied. Its advantages are high sensitivity and high correctness.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI
Methods for treatment of HIV and other infections using a T cell or viral activator and anti-retroviral combination therapy
InactiveUS20050208049A1BiocideOrganic active ingredientsPeripheral blood mononuclear cellCD4 antigen
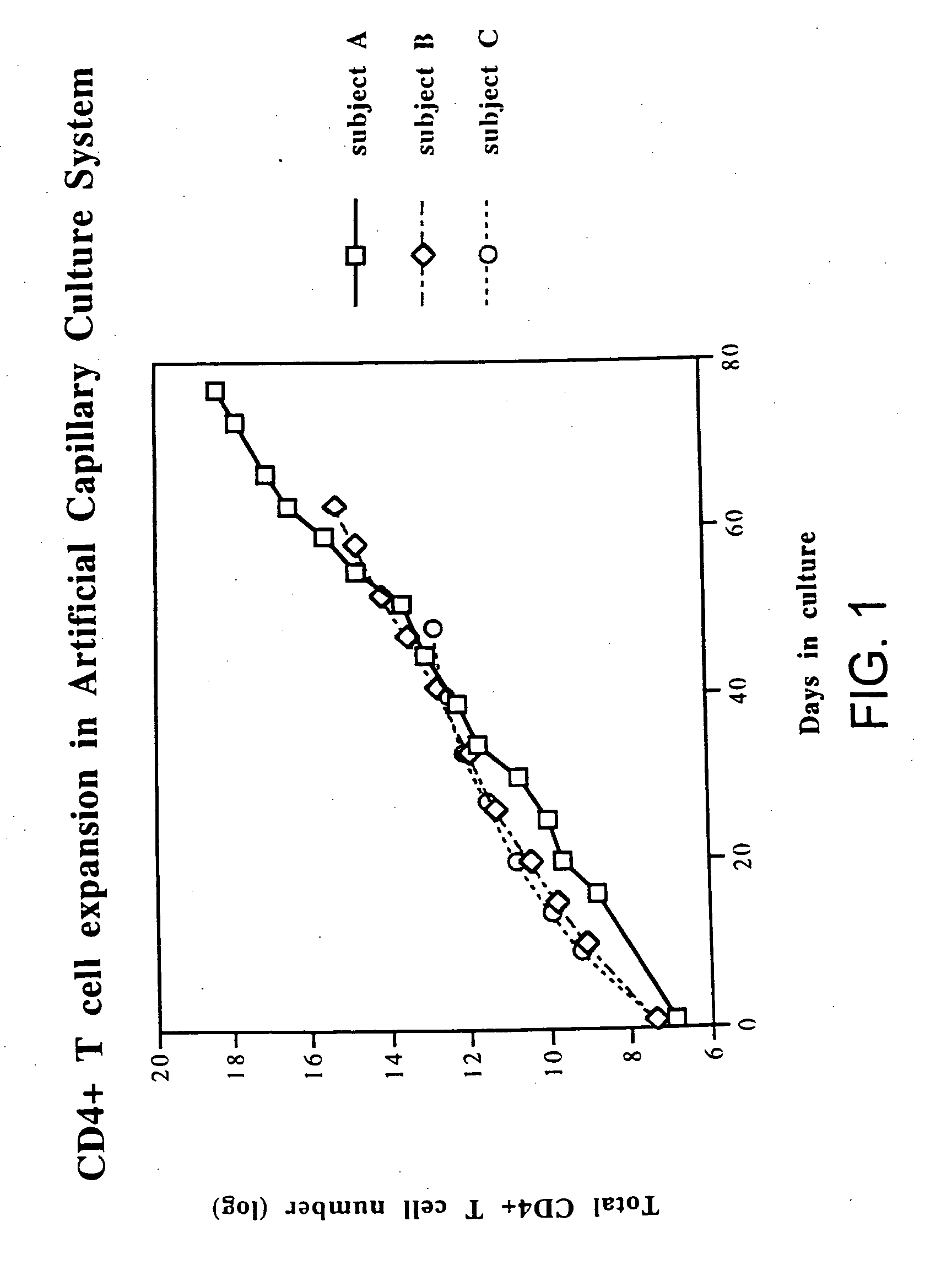
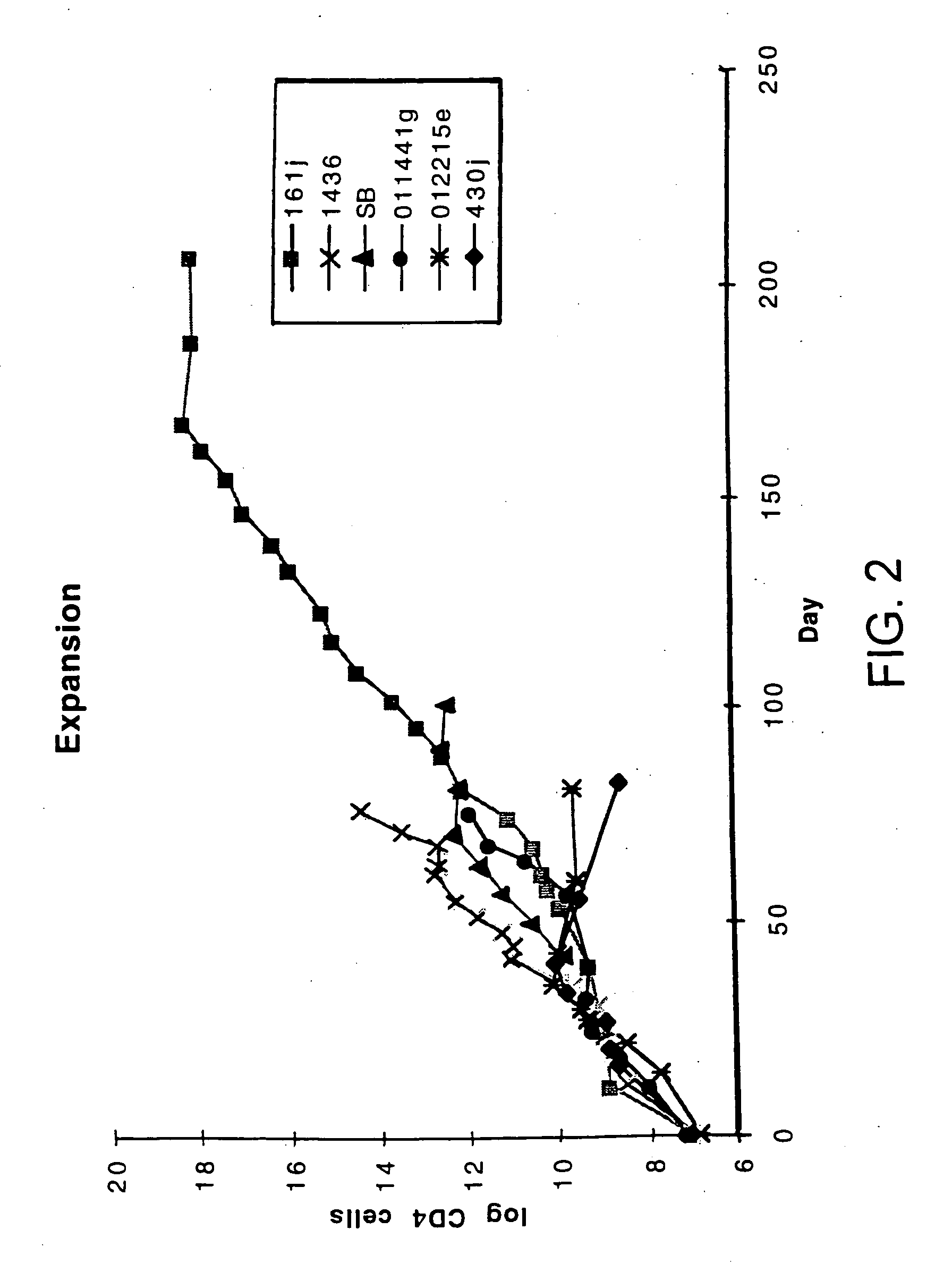
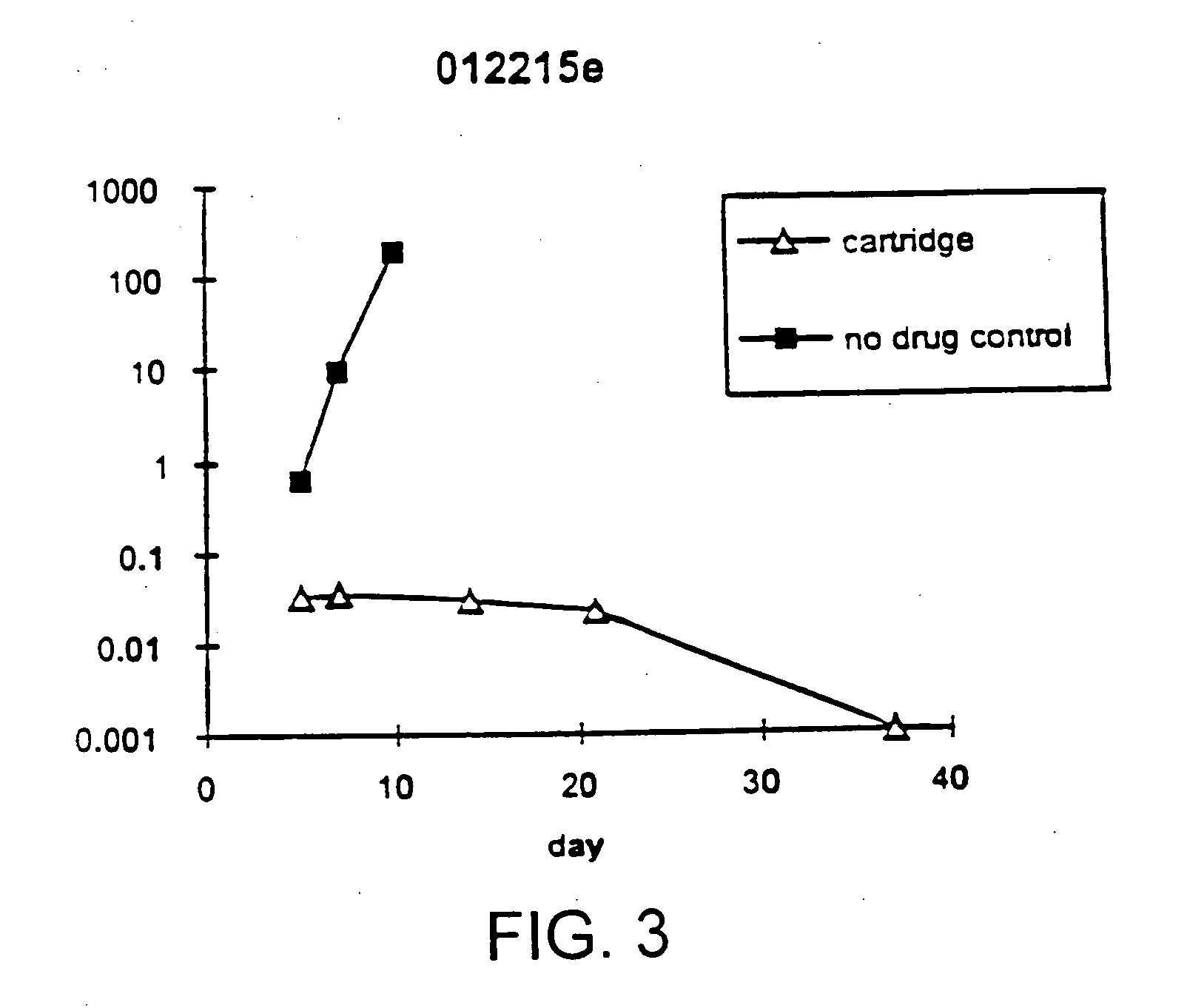
Disclosed is a method for treating infection with a pathogen. The method involves administration of: (1) a substance which induces active pathogen replication in a cell latently infected with HIV and (2) an anti-pathogen drug. Also disclosed are methods for expanding CD4+ T cells from peripheral blood mononuclear cells isolated from human subjects in the presence of an antiretroviral drug and for treating HIV infection by infusing the expanded CD4+ cells into HIV-infected patients.
Owner:WONG JOHNSON T
Composition and methods used during Anti-hiv treatment
InactiveUS20150342970A1Restoration of the normal phenotypeSymptoms improvedBiocideMetabolism disorderPremature agingSide effect
This invention relates to a composition comprising an anti-HIV treatment and a treatment for side effects of said anti-HIV treatment in an HIV-infected patient. This invention is, for example, very useful in the treatment of side effects caused by certain anti-HIV treatments, for example premature aging and lipodystrophy, which can be caused by protease inhibitors or reverse transcriptase inhibitors. The composition of this invention includes at least one hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, at least one farnesyl-pyrophosphate synthase inhibitor, and at least one anti-HIV agent. One of the processes for treating an HIV-infected patient includes, in any order, the following steps: (i) administration of a mixture including at least one hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor and at least one farnesyl-pyrophosphate synthase inhibitor and (ii) administration of an anti-HIV agent, in which the administrations are concomitant, successive or alternative.
Owner:UNIV DAIX MARSEILLE
Methods for treatment of HIV and other infections using a T-cell or viral activator and anti-retroviral combination therapy
Disclosed is a method for treating infection with a pathogen. The method involves administration of: (1) a substance which induces active pathogen replication in a cell latently infected with HIV and (2) an anti-pathogen drug. Also disclosed are methods for expanding CD4+ T cells from peripheral blood mononuclear cells isolated from human subjects in the presence of an antiretroviral drug and for treating HIV infection by infusing the expanded CD4+ cells into HIV-infected patients.
Owner:WONG JOHNSON T
Use of docosahexanoic acid as active substance for the treatment of lipodystrophy
ActiveUS20060178436A1Avoid toxicityBiocideElcosanoid active ingredientsMicroorganismDHA - Docosahexaenoic acid
Use of an extract of animal, plant or microorganism produced origin comprising docosahexaenoic acid as active substance for the manufacture of a medicament for the treatment of lipodystrophy in a mammal. Said treatment is effective and overcomes the disadvantages of current lipodystrophy treatments in HIV infected patients.
Owner:PROYECTO EMPRESARIAL BRUDY
Method for making an HIV vaccine
InactiveUS20030198941A1Viral antigen ingredientsMicrobiological testing/measurementEpitopeHIV Proteins
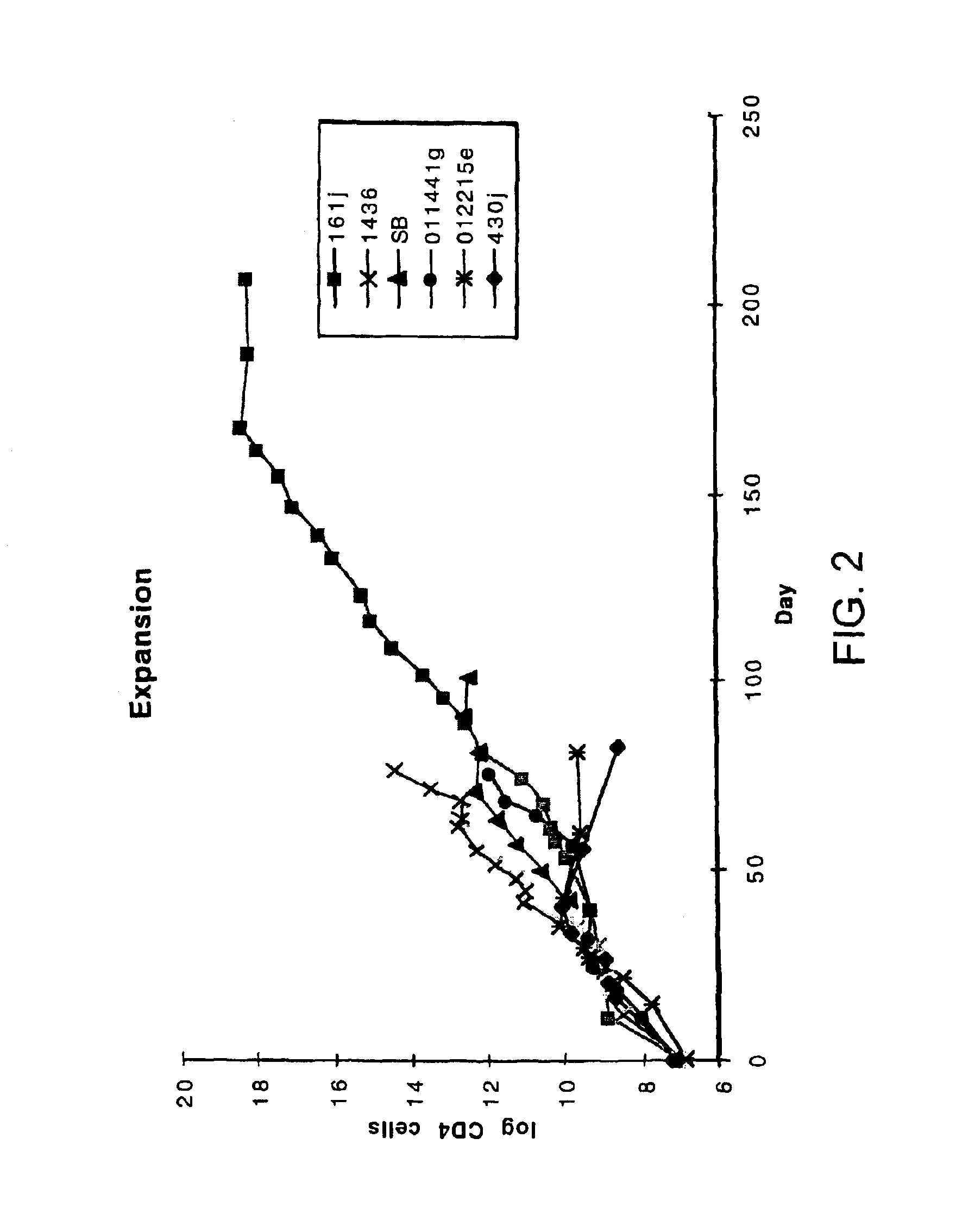
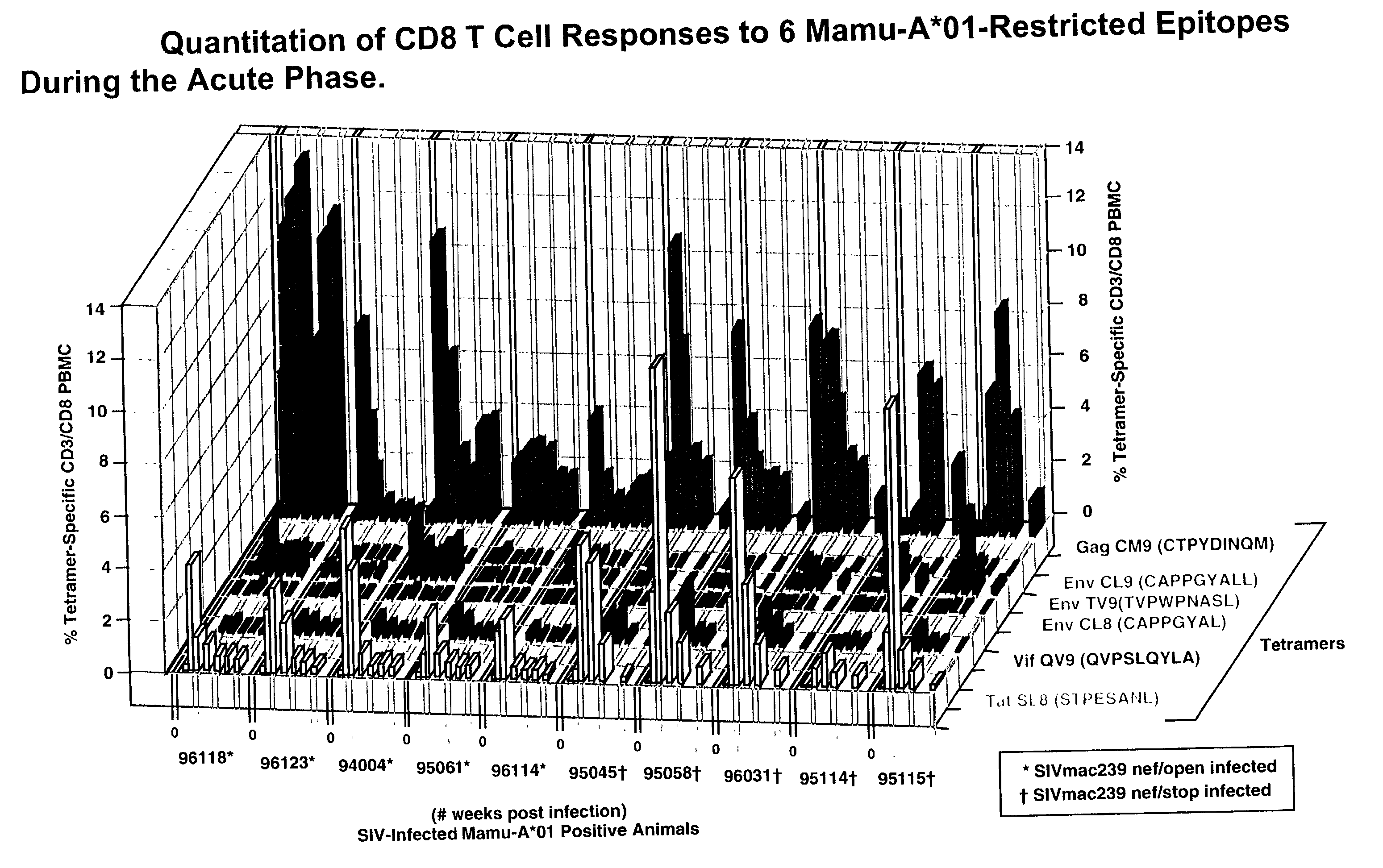
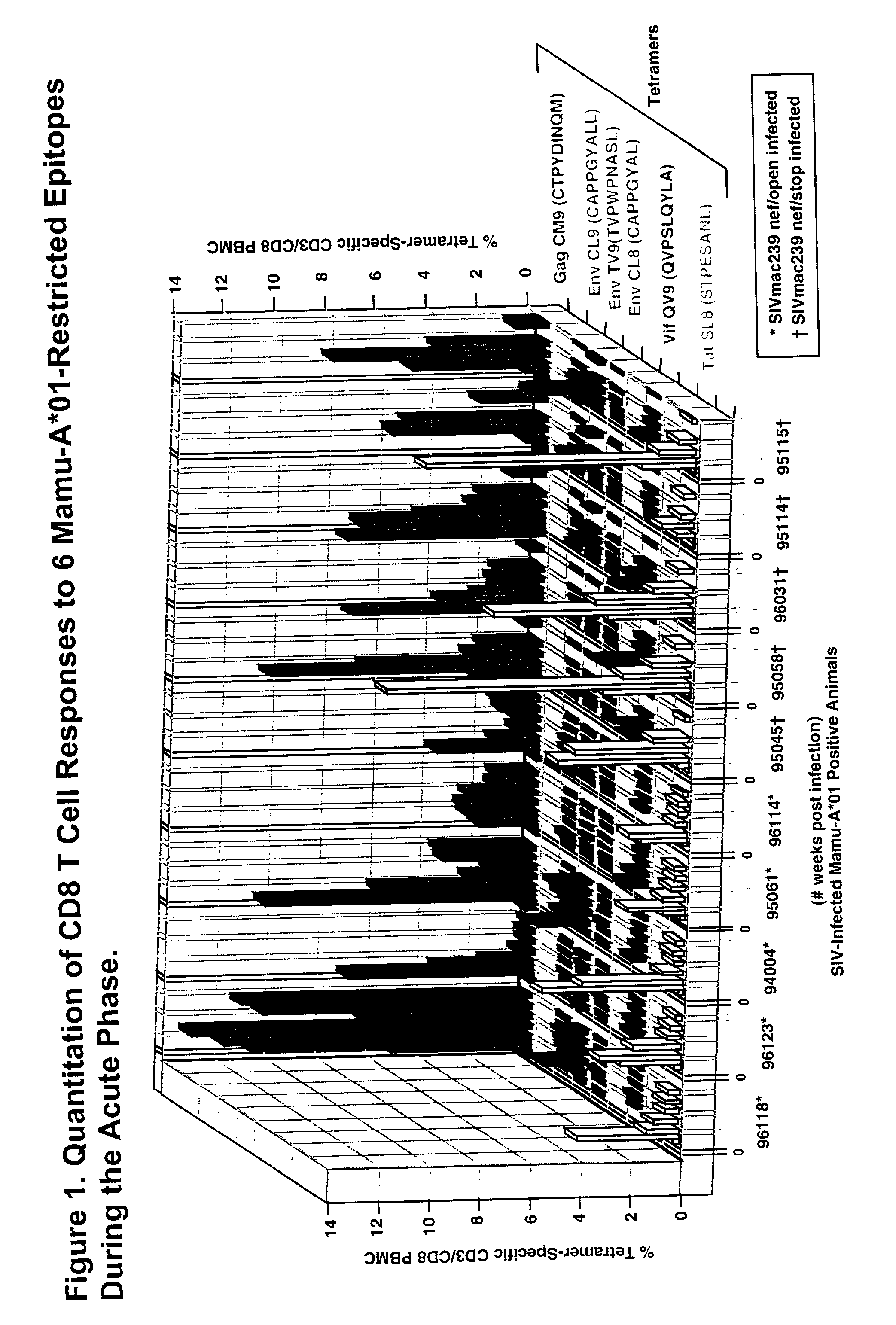
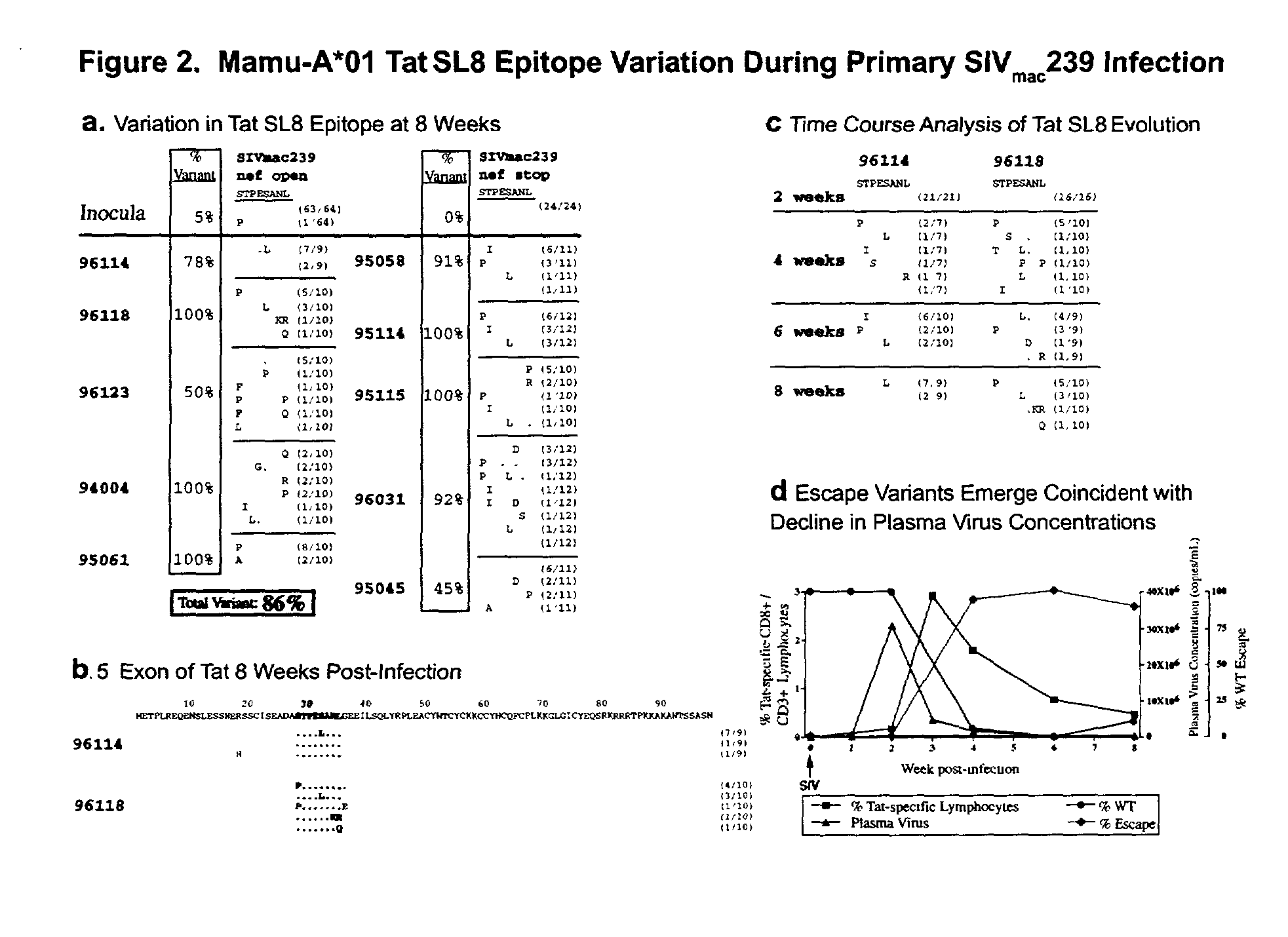
A method of identifying at least one CTL-inducing epitope from HIV protein is disclosed. In one embodiment, the method comprises the steps of (a) examining the nucleic acid sequence encoding at least one HIV protein from at least one HIV-infected patient, wherein the sequence encoding the expressed protein is examined in the first six months after infection, to identify at least one region of the HIV protein that is variable as compared to the sequence of the protein at an earlier time point in infection, wherein the variable region indicates a CTL-inducing epitope, and (b) confirming that an immune response directed against the CTL-inducing epitope is capable of selecting for viral escape variants during the acute or periacute phase of HIV infection is of high avidity.
Owner:WISCONSIN ALUMNI RES FOUND
Antiviral water-soluble product with anti-HIV effect, based on ionic silver and methylene blue compound; method of its production and examples of HIV-infected patients' treatment
A new antiviral water-soluble pharmacological product with anti-HIV effect, is based on ionic silver and methylene blue compound. The new substance is produced in the form of water-soluble powder that can be used for the preparation of finished dosage forms for treating viral infections, especially HIV infection. The product has a potential ability to radically suppress the infectious agents without leading to any mutations and resistance of viruses. The composition of the new product proposed under the conventional name Argothiazin-A™ corresponds to the chemical formula: C20H24AgN3O4S.
Owner:SVETLIK HARVEY E +5
Combinations of a pyrimidine containing nnrti with rt inhibitors
The present invention concerns combinations of a pyrimidine containing NNRTI with nucleoside reverse transcriptase inhibitors and / or nucleotide reverse transcriptase inhibitors useful for the treatment of HIV infected patients or for the prevention of HIV transmission or infection.
Owner:JANSSEN SCI IRELAND UC
Use of docosahexanoic acid as active substance for the treatment of lipodystrophy
ActiveUS8202906B2Avoid toxicityBiocideElcosanoid active ingredientsMicroorganismDocosahexaenoic acid
Use of an extract of animal, plant or microorganism-produced origin comprising docosahexaenoic acid as active substance for the manufacture of a medicament for the treatment of lipodystrophy in a mammal. The medicament is administered to a patient who is concomitantly receiving a highly active anti-retroviral therapy (HAART). The treatment is effective and overcomes the disadvantages of current lipodystrophy treatments in HIV-infected patients.
Owner:PROYECTO EMPRESARIAL BRUDY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com