Use of T cell comprising chimeric antigen receptor (CAR) modification for preparing cell drug
A chimeric antigen receptor and antigen technology, applied in the field of biomedicine, can solve the problems of HIV amplification, high cost, inability to target or eliminate latent HIV virus, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
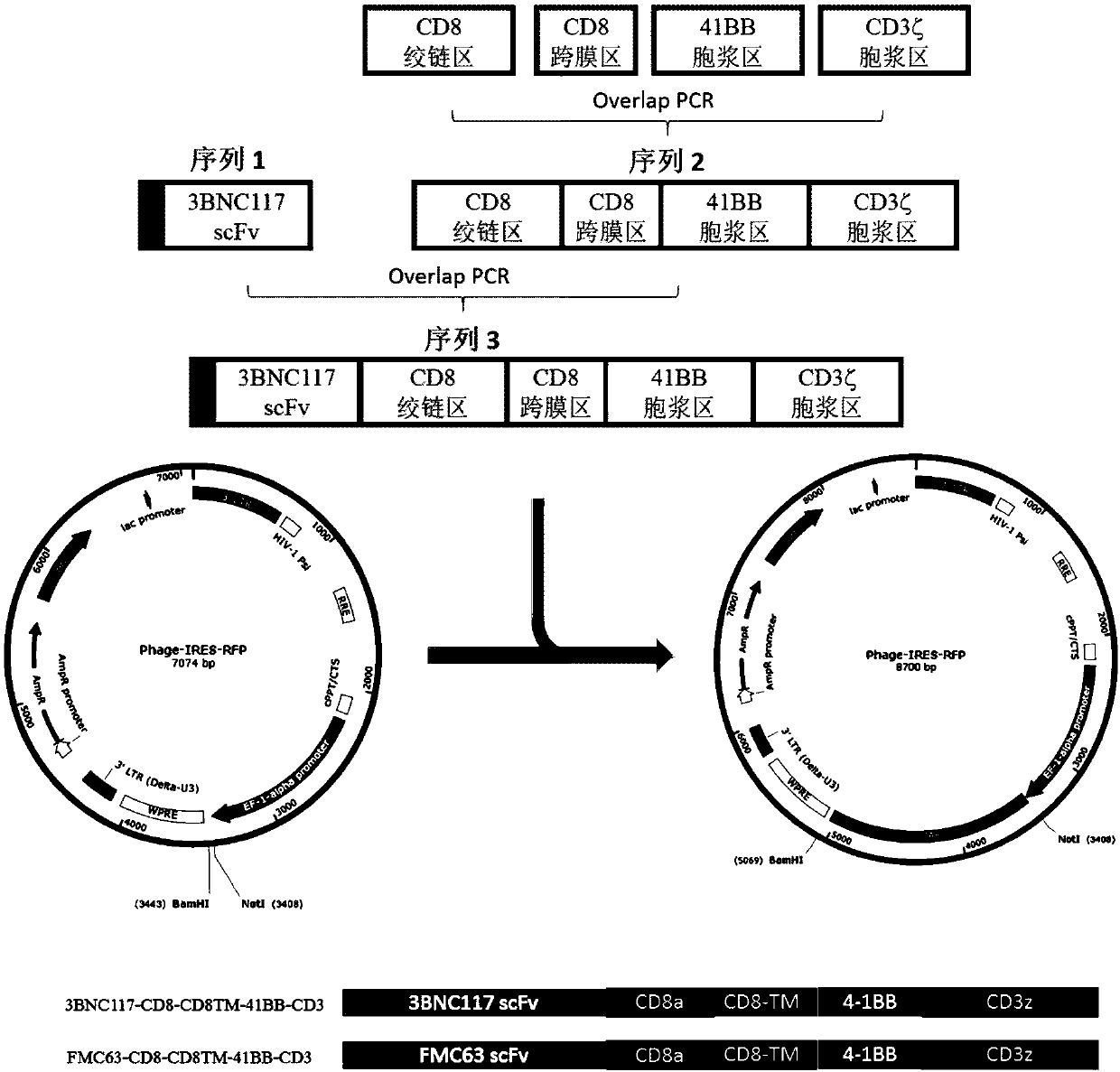
[0065] Example 1, Construction of 3BNC117-41BBCD3ξ-CAR
[0066] Prepare a variety of 3BNC117scFv, and generate CAR based on these scFv, the specific process is as follows:
[0067] (1) Preparation of various 3BNC117scFv
[0068] 1. Based on the nucleotide sequence of 3BNC117, synthesize antibody heavy chain (VH) and light chain (VL) sequences, and design Overlap PCR primers based on the sequences.
[0069] 2. Use DNA polymerase to perform PCR amplification to amplify the VL fragment and VH fragment of 3BNC117. After completion, perform PCR product recovery and DNA agarose gel electrophoresis to verify whether the PCR band size is correct.
[0070] 3. Using the correctly identified 3BNC117 VL and VH fragments as templates, Overlap PCR primers were used to perform overlap PCR amplification. After completion, DNA agarose gel electrophoresis was performed to verify the correct bands, and the correct bands were cut and recovered to obtain 3BNC117scFv;
[0071] 4. The 3BNC117scFv ...
Embodiment 2
[0109] Example 2. Preparation of CAR-T cells (exemplarily using phage-3BNC117scFv-CAR as described in SEQ ID NO: 14)
[0110] (1) Lentivirus packaging, concentration and titer detection
[0111] 1.1 Resuscitate and adjust the state of 293T cells, re-plating about 24 hours before transfection, and it is advisable to pack and transfect cells with a confluence of more than 70%;
[0112] 1.2 Plasmids psPAX2, pMD2.G and CAR-expressing lentiviral plasmid phage-3BNC117scFv-CAR required for the transfection virus packaging system. Before transfection, replace the medium with serum-free medium. For a 15cm cell culture dish, proceed as follows operate:
[0113] Add 60μl PEI dropwise to 1ml DMEM, let stand for 5 minutes,
[0114] Take another tube to join
[0115] phage-3BNC117scFv-CAR 15μg
[0116] psPAX2 10 μg
[0117] pMD2.G 5 μg
[0118] Add DMEM to 1ml,
[0119] Add the PEI mixture dropwise and mix it into the packaging plasmid mixture, and let it stand for 20 minutes;
[01...
Embodiment 3
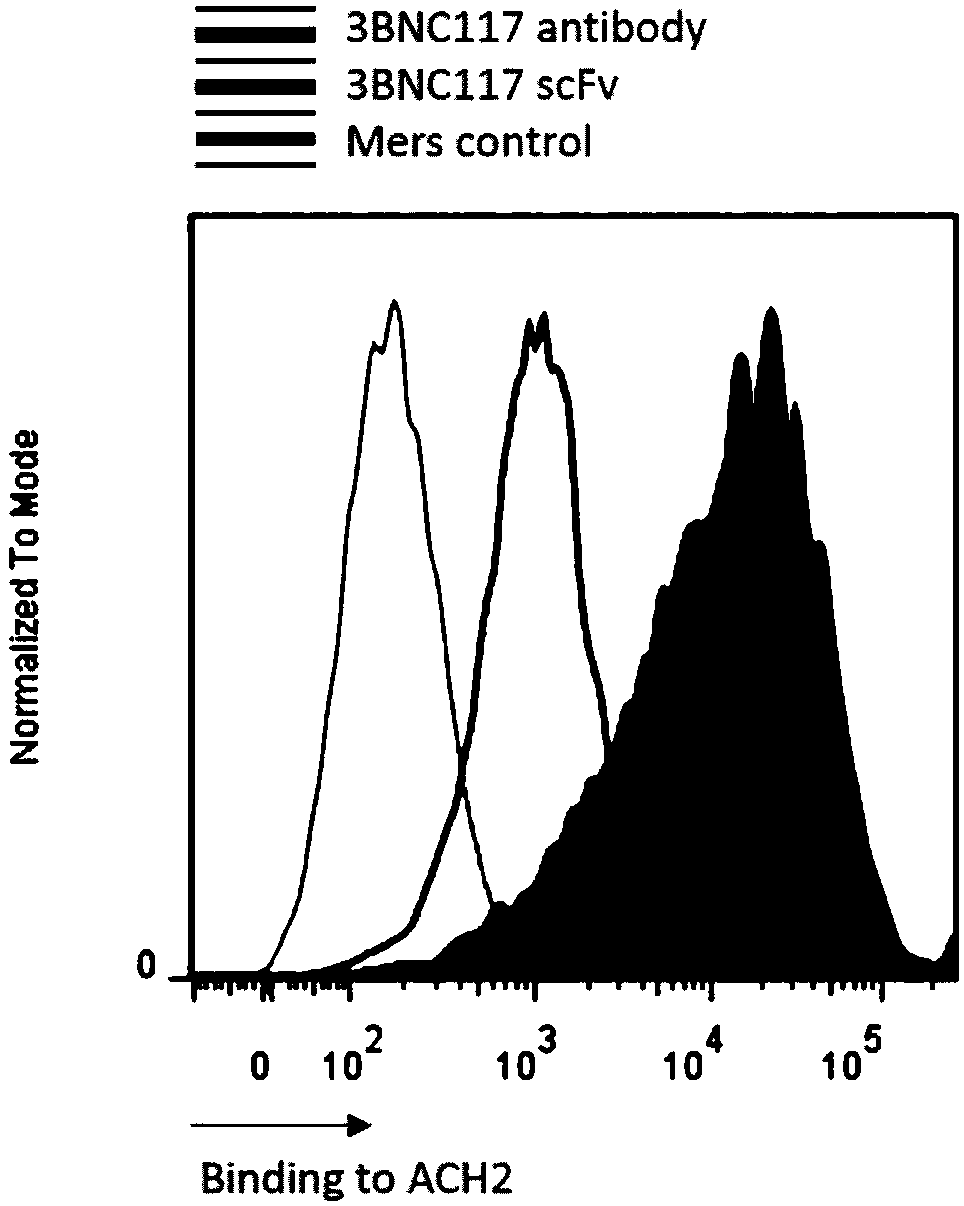
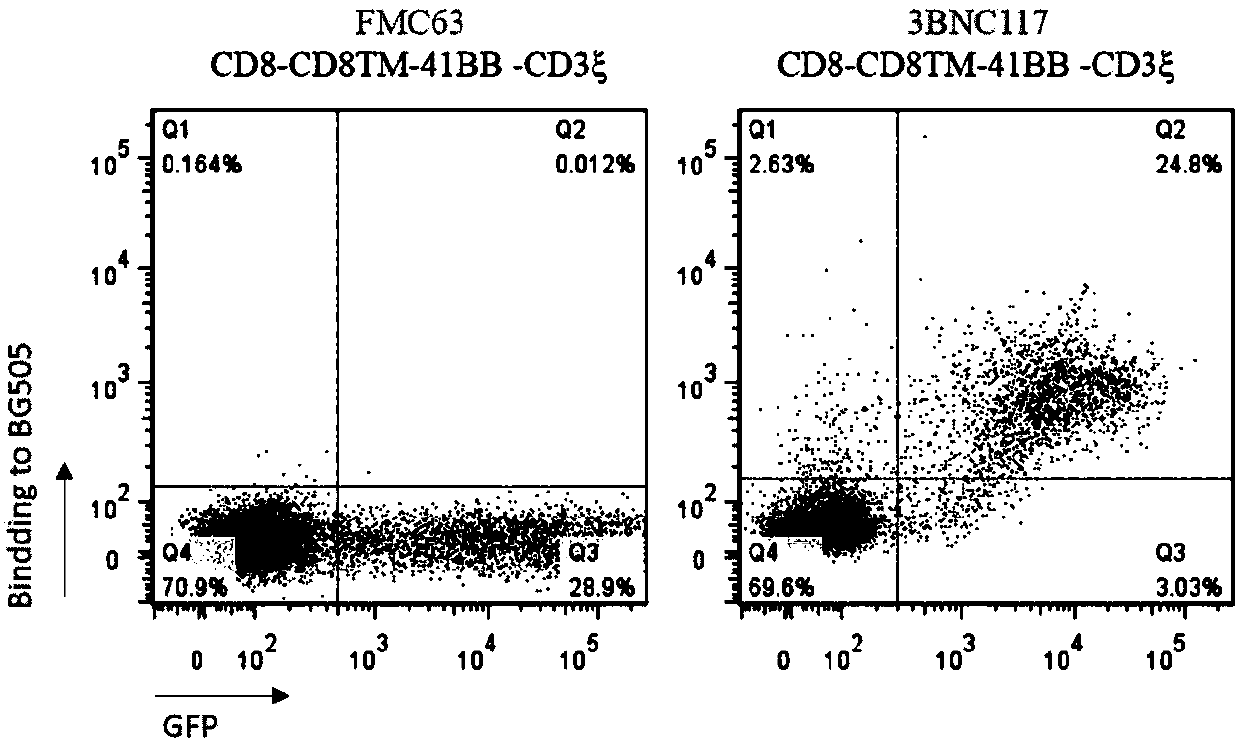
[0131]Example 3, HIV envelope protein and 3BNC117-41BBCD3ζ-CAR specific recognition and binding verification experiments
[0132] In this experiment, to study whether the HIV envelope protein specifically recognizes and binds to 3BNC117-41BBCD3ζ-CAR, 3BNC117-41BB CD3ζ-CAR-GFP was first transfected into 293T cells, and after 24 hours, the HIV envelope with his tag Protein gp120 was incubated with anti-his PE-labeled antibody, and the combination of HIV envelope protein by CAR-GFP positive cells was observed by flow cytometry.
[0133] (1) Cells and reagents
[0134] 1. Experimental cells: 293T cells, purchased from ATCC, and cultured in the cell experiment platform of Tsinghua University.
[0135] 2. HIV envelope protein for experiment: The HIV envelope protein gene (CNE54) from Chinese HIV-infected patients was cloned into a eukaryotic vector, transferred into 293f cell culture for production, and purified by the AKTA protein purification system.
[0136] 3. The anti-his PE-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com