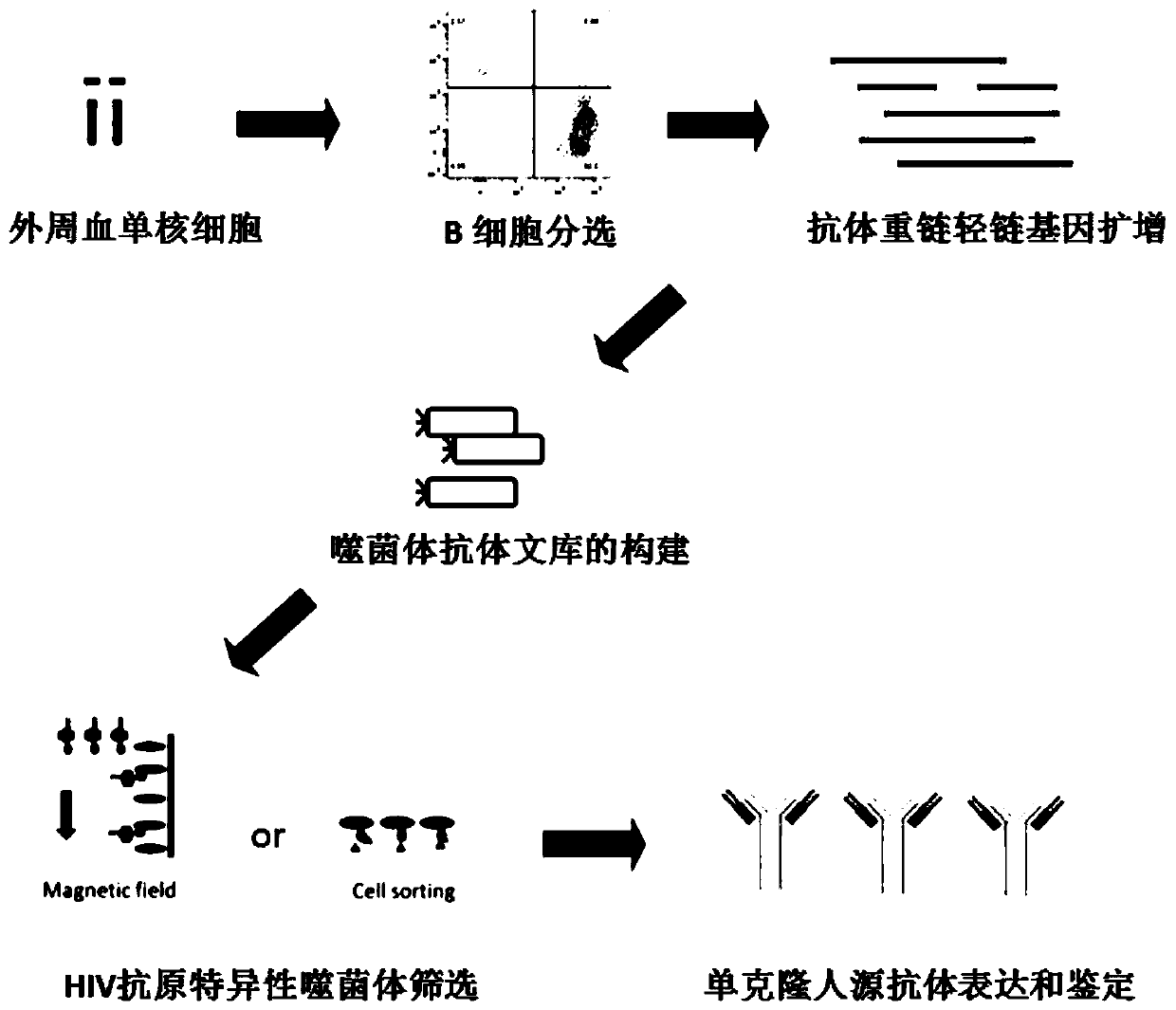
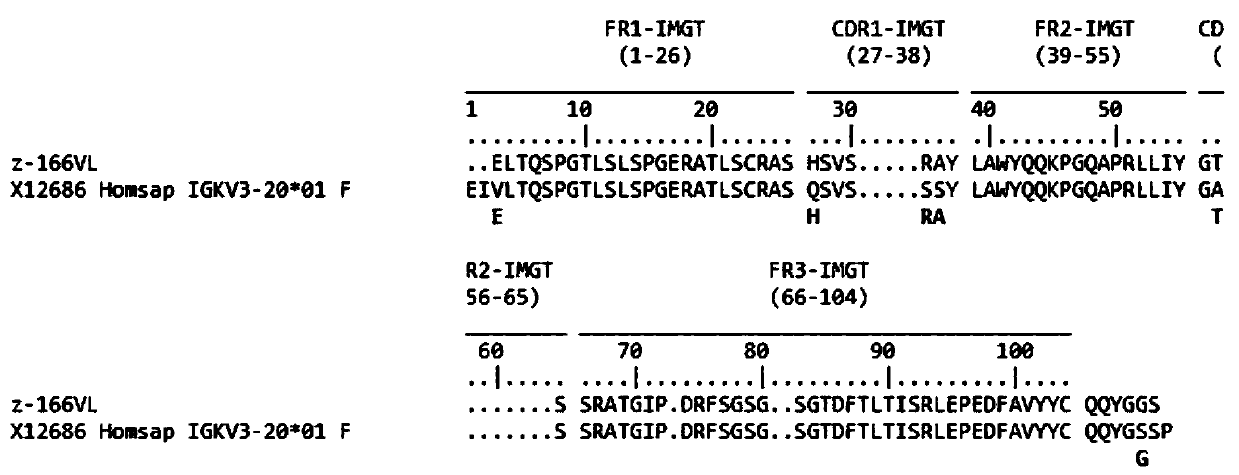
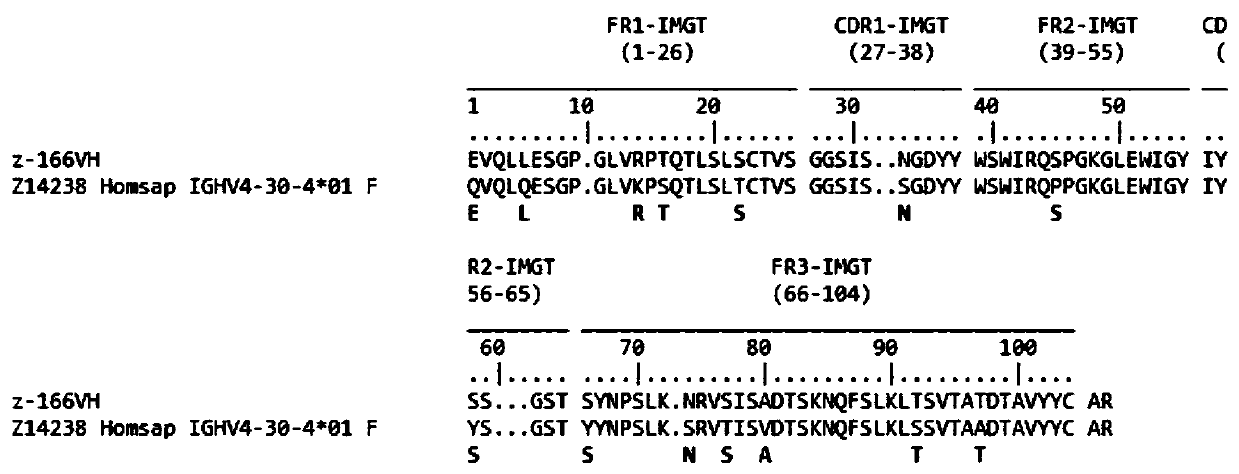
Human anti-HIV gp 120 specific antibody Z166 and application method thereof
A Z166, specific technology, applied to human anti-HIV gp120 specific antibody Z166 and its application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Example 1: Preparation of Human Anti-HIVgp120 Specific Antibody
[0025] 1. Antigen labeling.
[0026] A plasmid (pcDNA) containing the full-length envelope protein (Accession NO.EU123924) of HIV Sf162 isolate was expressed from 293F (Thermo Fisher Ltd, CAT#R79007) cells TM 3.1 + , Thermo Fisher Ltd, CAT#V79020), the gp140 trimer (SF162) in the 293F supernatant was purified by nickel column purification. Dilute the trimer protein to 0.5-2 mg / ml with PBS, then use Thermo Fisher's biotin (CAT#21335) to label the gpl40 protein according to its kit procedure (the ratio of the number of molecules is protein:biotin=1: 20~100), incubate at room temperature in the dark for 0.5~2h, then centrifuge 4~6 times at 8000g with a 10kD centrifugal semi-permeable column (MerckMillipore Ltd, CAT#UFC501096), supplement with sterile PBS, and remove excess biotin molecules , the labeled gp120 protein molecules will be used to screen antigen-specific antibodies.
[0027] 2. Antigen-specif...
example 2
[0037] Example 2: Neutralizing activity of monoclonal antibody against HIV-1 pseudovirus
[0038] Pseudovirus packaging and antibody neutralization activity detection system according to the relevant literature "Reconstitution and characterization of antibody repertoires of HIV-1-infected"eliteneutralizers"." Antiviral Res 118:1-9. The description proceeds. Briefly described as follows, 293T cells are used to co-transfect PNL4-3 plasmid (containing VSV virus backbone and containing luciferase gene) and HIV-1 envelope protein particles to package HIV-1 pseudovirus. After culturing for 72 h, the virus supernatant was collected and the virus titer was detected. After infecting TZM-bl cells in a 96-well plate with an appropriate amount of virus and antibody gradient dilution, the cells were lysed after 72 hours, and the luciferase activity in the lysed cells was detected by a luciferase luminescence detection system (Promega Ltd) to determine the antibody Inhibitory effect on ...
example 3
[0039] Example 3: Detection of antibody-dependent cell-mediated cytotoxicity (ADCC) activity of monoclonal antibodies
[0040] TF228 cells were cultured in DMEM medium (see references (Puri, Hug et al.1998) for cell sources and characteristics), the cell density was about 90%, the cells were digested with trypsin, washed twice with PBS and resuspended, followed by the PKH67 labeling kit (Sigma, PKH67GL-lKT) process to mark TF228 cells, and adjust the cell density to be 106 / ml spare. Dilute the antibody to be tested in gradient with DMEM medium, mix with 100μl labeled TF228 cells, and place at 37°C, CO 2 Incubate in a cell culture incubator with a concentration of 5% for 15 minutes; add 100 μl to a density of 2x10 7 / ml of freshly isolated healthy human peripheral blood mononuclear cells (PBMC), mixed and placed at 37 ° C, CO 2 The concentration was 5% and cultured in a cell incubator for 4 hours; 1 μl 7AAD (ThermoFisher Ltd, Cat# 302232) was added to each reaction, mixed an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com