Compositions and methods for treating hiv/aids with immunotherapy
A technology of combining structural domains and chimeric antigen receptors, applied in biochemical equipment and methods, chemical equipment and methods, for targeting specific cell fusion, etc., can solve problems such as reducing therapeutic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
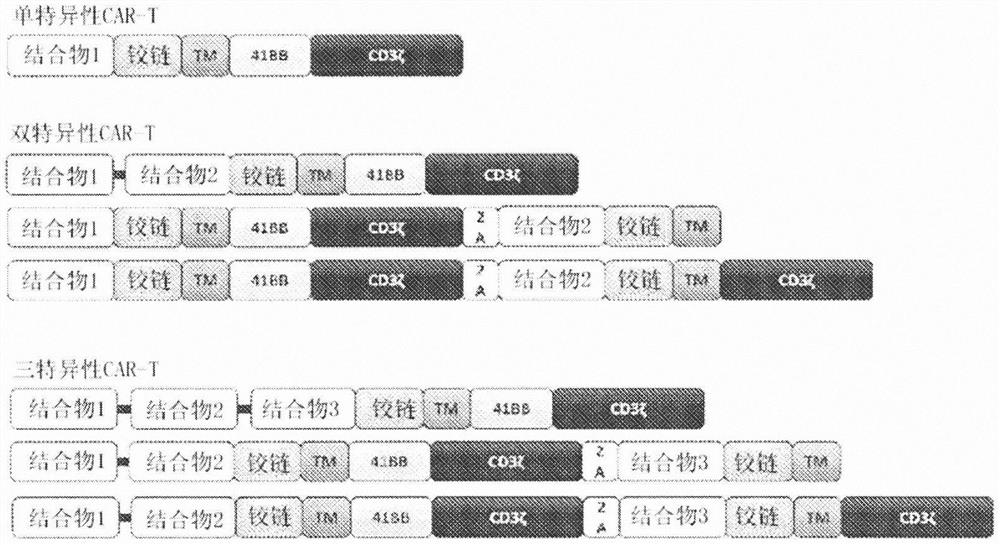
[0473] Assembly of HIV-specific binders into functional CAR molecules
[0474] This example describes the general method used to construct monospecific, bispecific and trispecific anti-HIV CARs comprising mD1.22, m36.4 and C46 peptides, and how to express these CARs on the surface of primary T cells.
[0475] Materials and methods:
[0476] Generation of lentiviral vector constructs
[0477] The CAR antigen-binding domain sequence is derived from published sequences (Chen et al., J. Virol. 2014; 88: (2) 1125-1139; Chen et al., Antiviral Research 2010; 88: (1) 107-115; Egerer et al., Molecular Therapy 2010;19:(7)1236-1244) and were synthesized by ATUM (formerly DNA2.0; Newark, CA) or IDT Technologies (IA, Coralville). Synthetic gene fragments were subcloned in-frame onto an MSCV promoter-based lentiviral backbone comprising the CD8 linker / hinge, CD8 transmembrane domain, 41-BB, and CD3ζ signaling domain. For bicistronic bispecific and trispecific CAR constructs, the cleavabl...
Embodiment 2
[0491] Novel bispecific and trispecific anti-HIV CARs potently destroy HIV envelope targets
[0492] This example describes the functional characterization of anti-HIVCAR as determined by a highly sensitive luciferase-based cytotoxicity assay. Additionally, T cell activation was determined by quantification of cytokine secretion in the absence and presence of HIV-envelope expressing target cells.
[0493] Materials and methods:
[0494] Cell Lines Used for Functional Characterization
[0495] The 293T cell line was engineered to stably express the single-chain full-length HIV envelope protein (293T-Env) and was generously provided by Dr. Dimiter Dimitrov (NCI, Fort Detrick, MD). Briefly, 293T-Env cells were grown in Dulbecco's modified eagle medium (DMEM) in the presence of 10% fetal bovine serum and 60 μg / ml zeocin to maintain selection . To generate luciferase-expressing cells, 293T-Env cells were transduced with a lentiviral vector containing the firefly luciferase gene...
Embodiment 3
[0516] Bispecific and trispecific anti-HIV duoCAR-T cells broadly and potently eliminate HIV-infected PBMCs in vivo and in vitro
[0517] This example interrogates the anti-HIV CAR-T cell killing potency and the susceptibility of CAR-T cells to HIV-1 infection after in vitro and in vivo challenge of PBMCs infected with different Env-IMC-LucR HIV-1 viruses.
[0518] Materials and methods:
[0519] In vitro potency of an anti-HIV CAR using a replication-competent Env-IMC-LucR molecular clone to infect donor-matched PBMCs
[0520] Inhibition of HIV-1 infection was investigated using a replication-competent HIV-1 molecular clone containing the desired heterologous HIV-1 envelope upstream of the Renilla luciferase ORF in frame (Env-IMC-LucR) . Infectious clones were generated as previously described (Edmonds et al., Virology 2010, 408: 1-13). HIV-1 infection of PBMC or CD4 + Following T cells, Renilla luciferase expression was used as a highly sensitive and quantifiable measure o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com