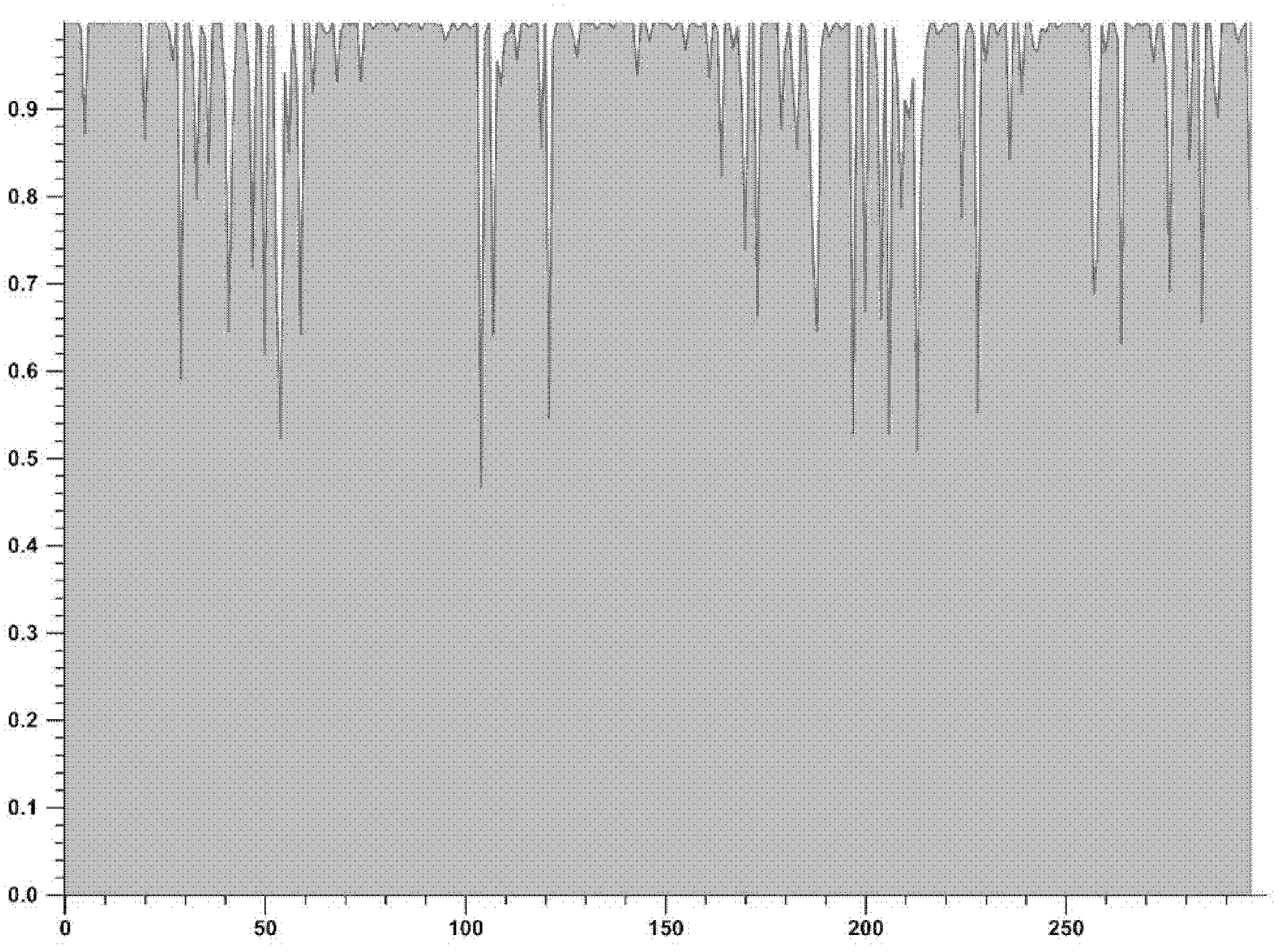Primer composition for identifying HIV (human immunodeficiency virus) in assistance mode and application thereof
A primer composition and a technology for identifying people, which can be applied in the field of assisting HIV identification, and can solve the problems of blood safety threat, high risk of transmission, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, the design of primer composition
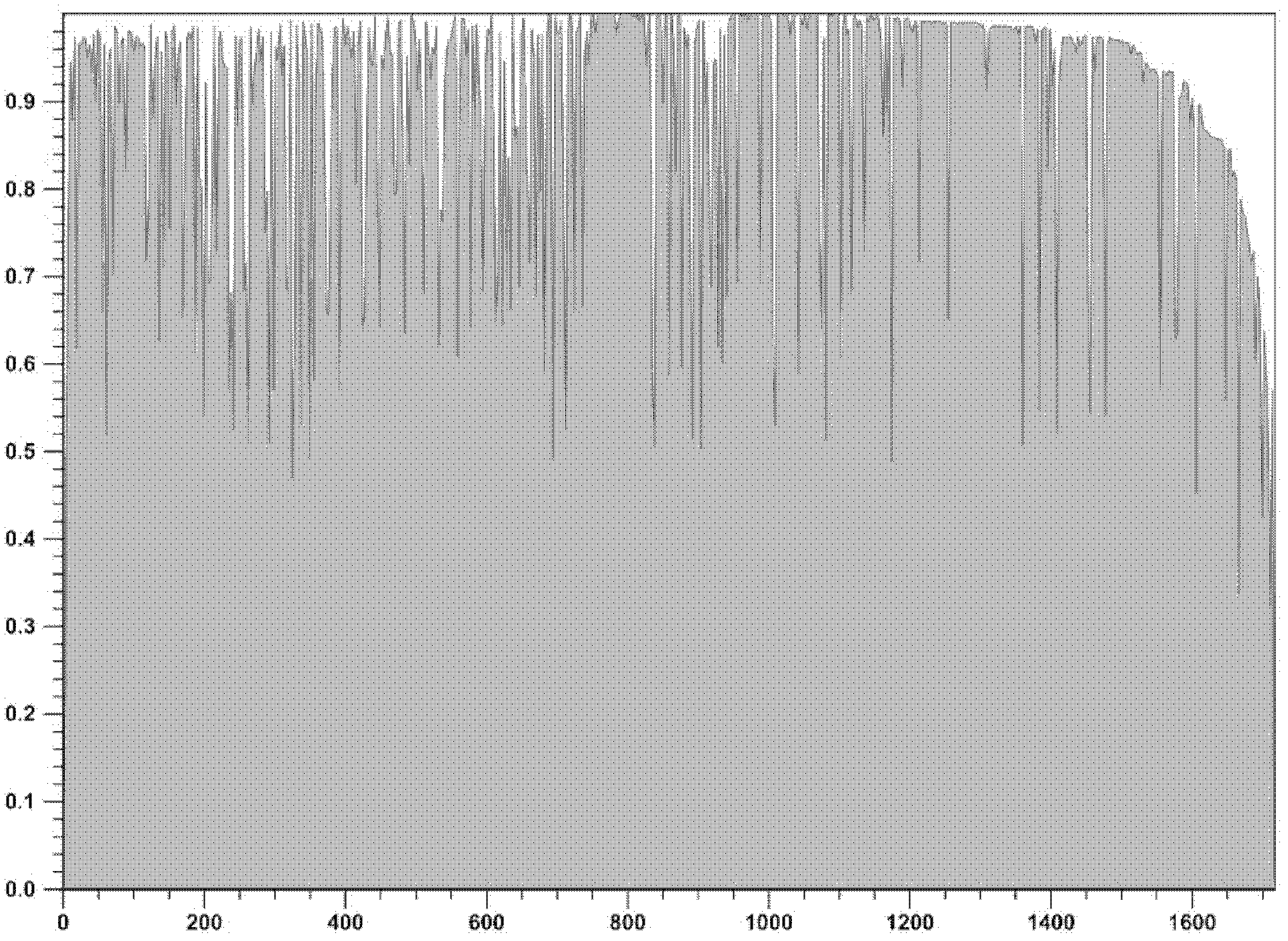
[0022] Take advantage of Vector NTI Advance TM 10 The analysis software scans the conserved regions of a total of 1582 protease region and reverse transcriptase region sequences (sequence composition as shown in Table 1) from HIV-infected persons, and observes the degree of variation of the pol region genes of different subtypes. The scanning results showed that the gene conservation of the protease region in the pol region was better than that of the reverse transcriptase region.
[0023] Table 1 Gene sequence composition of HIV-1 pol region
[0024]
[0025] For the results of scanning the conserved region of the protease region, see figure 1 . For the results of scanning the conserved region of the reverse transcriptase region, see figure 2 .
[0026] A group of primers (named as primer composition HIV-CN-II) are designed based on sequence analysis results, by primer HIV-CN-II-1, primer HIV-CN-II-2, primer HIV...
Embodiment 2
[0029] Embodiment 2, the sensitivity of primer composition
[0030] 1. Experimental samples
[0031] The plasma samples of 41 clinically confirmed HIV-infected persons (informed consent volunteers) were used as experimental samples, among which 17 were infected with HIV-1B' subtype strains, and 12 were infected with HIV-1CRF01-AE subtype 12 patients were infected with HIV-1B / C recombinant subtype strains.
[0032] 2. Application of primer composition
[0033] The plasma of each patient in step 1 was subjected to the following experiments:
[0034] 1. Extract total RNA.
[0035] 2, with the total RNA of step 1 as template, carry out RT-PCR (the first round of PCR amplification) with the primer pair that HIV-CN-II-1 and HIV-CN-II-2 form, obtain the first round of PCR amplification increase product.
[0036] The reaction system of the first round of PCR amplification is shown in Table 3.
[0037] Table 3 The reaction system of the first round of PCR amplification
[0038] ...
Embodiment 3
[0055] Embodiment 3, the specificity of primer composition
[0056] 1. Experimental samples
[0057] Five HTLV-infected patients who were clinically confirmed and positive for nucleic acid testing (volunteers who gave informed consent; numbers were HTLV25, HTLV26, HTLV36, HTLV37, HTLV38), and five HBV-infected patients who were clinically confirmed and positive for nucleic acid testing (informed consent volunteers). Volunteers; numbers are HBV-2, HBV-5, HBV-6, HBV-10, HBV-12), 7 HCV infected persons who were clinically confirmed and positive in nucleic acid test (informed consent volunteers; numbers are respectively HCV-5, HCV-71, HCV-75, HCV-76, HCV-93, HCV-7, HCV-56) as experimental samples.
[0058] 2. Application of primer composition
[0059] The plasma of each patient in Step 1 was subjected to the same experiment as Step 2 of Example 2.
[0060] The agarose gel of the second round of PCR amplification products is shown in Figure 5 . The amplified experimental samp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com