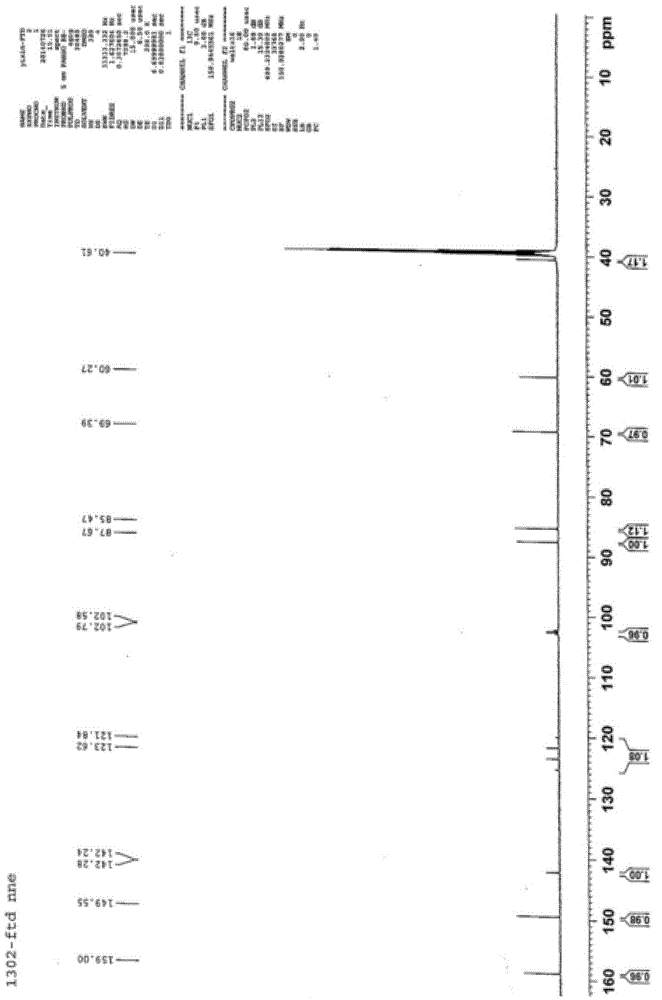
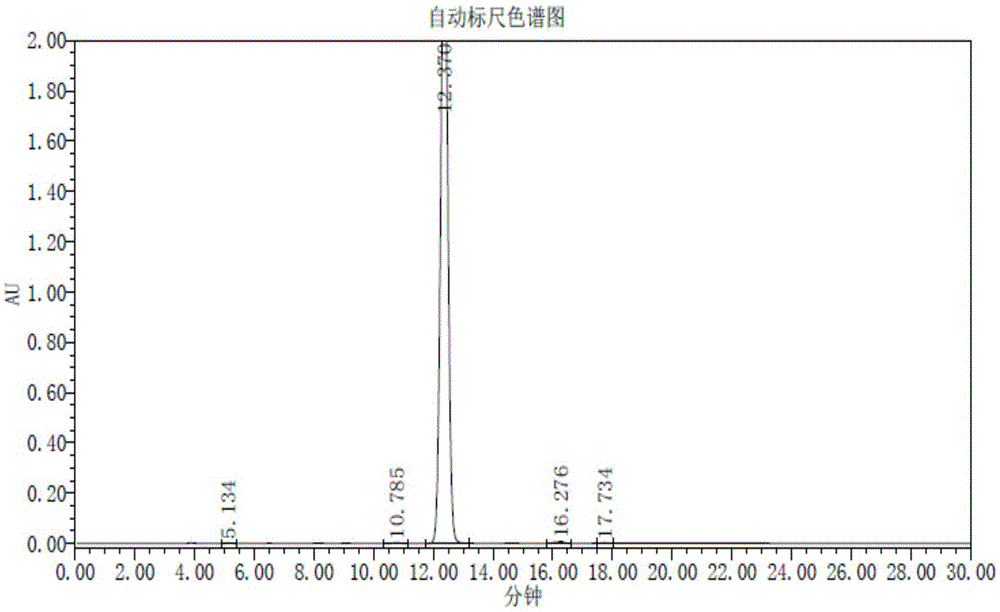
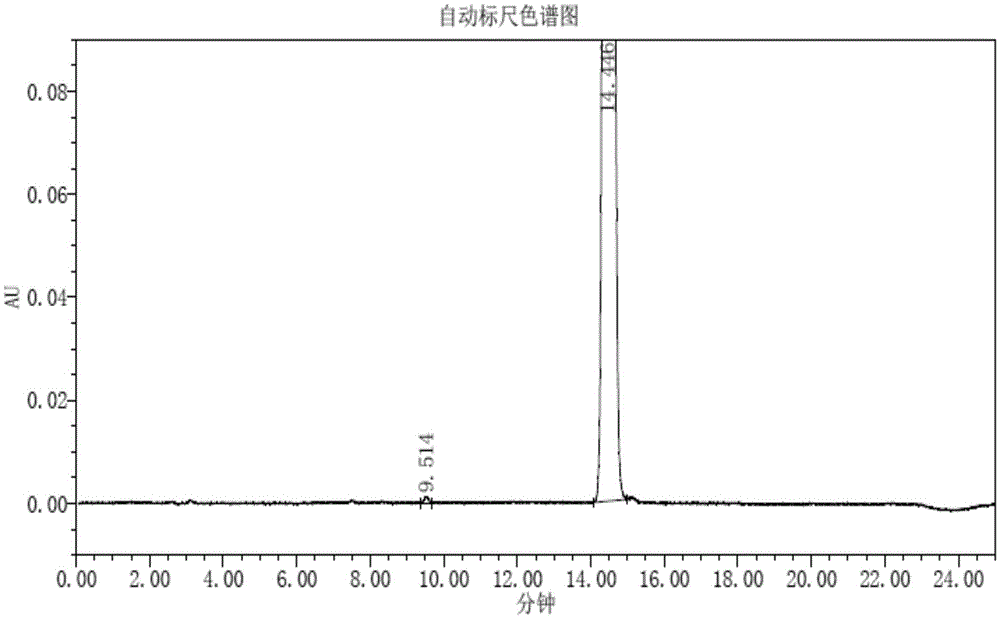
Trifluridine intermediate and preparation method of trifluridine
A technology for trifluridine and intermediates, applied in the field of drug synthesis, can solve problems such as being difficult to be applied in industrial production, and achieve the effects of controllable product quality, high purity and improved production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The invention provides a kind of preparation method of trifluridine intermediate, comprising the following steps:
[0038] A) under the effect of acidic resin catalyst, 1-chloro-2-deoxy-3,5-two-O-p-chlorobenzoyl-D-ribose and 5-trifluoromethyl-2,4-bis( After the condensation reaction of trimethylsilyloxy)pyrimidine, the trifluridine intermediate is obtained.
[0039] Described acidic resin is preferably one or more in Amberjet1500H cationic resin, Dowex50W-X2 resin and strongly acidic styrenic cation exchange resin, more preferably Amberjet1500H cationic resin, Dowex50W-X2 resin or strongly acidic styrenic cation exchange resin Resin, most preferably Dowex50W-X2 resin; The mass ratio of described 1-chloro-2-deoxy-3,5-two-O-p-chlorobenzoyl-D-ribose and acidic resin catalyst is preferably 1:(0.008 ~0.02), more preferably 1:(0.01~0.018), more preferably 1:(0.012~0.016), most preferably 1:(0.013~0.015); the 1-chloro-2-deoxy-3,5 The mass ratio of -di-O-p-chlorobenzoyl-D-rib...
Embodiment 1
[0061] 1. Preparation of 5-trifluoromethyl-2,4-bis(trimethylsilyloxy)pyrimidine
[0062] Weigh 2.40 kg of 5-trifluoromethyluracil into a 50 L reaction flask, add 14.8 L of HMDS, drop in 60 ml of TMSCl, and react at a temperature of 110° C. for 3 h. Distill under reduced pressure, collect the product of stable fraction (130 ℃, vacuum: 0.090mpa), obtain 3.66kg of 5-trifluoromethyl-2,4-bis(trimethylsilyloxy)pyrimidine, calculate the reaction yield The rate is 85.0%.
[0063] 2. Preparation of 1-(2'-deoxy-3,5-di-O-p-chlorobenzoyl-β-D-furanosyl)-5-trifluoromethyluracil
[0064]Add 3.66g of 5-trifluoromethyl-2,4-bis(trimethylsilyloxy)pyrimidine prepared in the above steps, 36.6mg of Dowex50W-X2 resin and 50mL of chloroform into a 100mL enamel reaction bottle, at 25°C Stir at a temperature of 10 min, and after the solid is basically dissolved, add 3.72 g of 1-chloro-2-deoxy-3,5-di-O-p-chlorobenzoyl-D-ribose to the mixed system, and stir for another 12 h. filter.
[0065] After th...
Embodiment 2
[0081] Preparation of 1-(2'-deoxy-3,5-di-O-p-chlorobenzoyl-β-D-furanosyl)-5-trifluoromethyluracil
[0082] Add 3.66 g of 5-trifluoromethyl-2,4-bis(trimethylsilyloxy)pyrimidine, 36.6 mg of Amberjet 1500H cationic resin and 50 mL of chloroform into a 100 mL enamel reaction bottle and stir at a temperature of 25°C After 10 minutes, after the solid was dissolved, 3.72 g of 1-chloro-2-deoxy-3,5-di-O-p-chlorobenzoyl-D-ribose was added to the mixed system, stirred for another 12 hours, and filtered.
[0083] After the reaction was completed, 20 mL of hydrochloric acid with a concentration of 1 mol / L was added to the system, stirred for 15 min, and 20 mL of water was added to the system again, after stirring for 15 min, the liquid was separated. Add 10 mL of saturated brine to the organic phase to wash once, add 2 g of anhydrous sodium sulfate to dry, filter, and then concentrate the organic solvent under reduced pressure, and add anhydrous ethanol (12 mL)-n-hexane (30 mL) mixed solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com