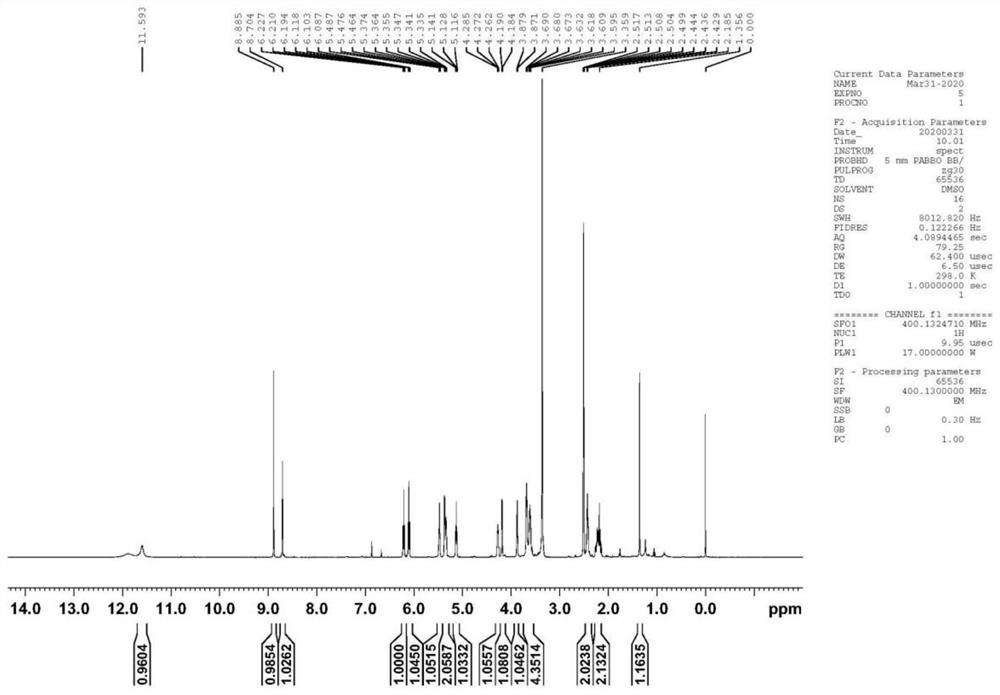
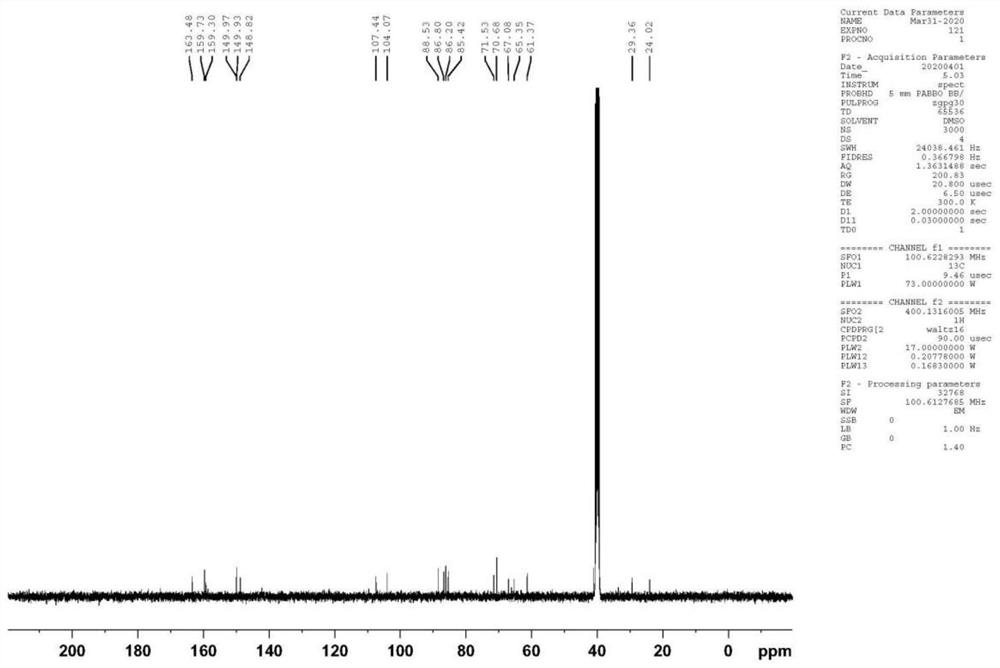
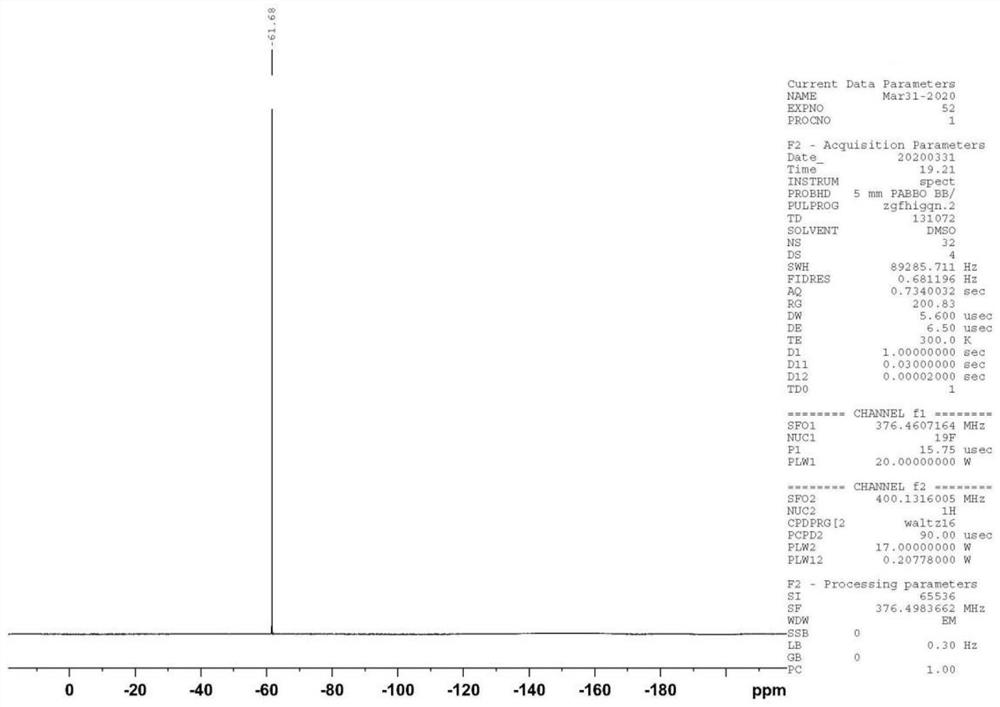
Trifluridine impurity compound and preparation method thereof
A technology of trifluridine and compound, applied in the field of pharmaceutical synthesis, can solve the problems such as impurity compound I and its synthesis method which are not reported in literature, and achieve the effects of short route, high product purity and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] At room temperature, compound SM-1 (17.81g, 0.05mol), trifluridine (41.47g, 0.14mol), and Novozyme435 (44.53g) were added to acetone (200ml), and the temperature was controlled at 25-30°C to react. After completion, add LiOH (3.60g, 0.15mol) purified water (20ml) solution at temperature control of 10-15°C. Concentrate under reduced pressure to dryness after deactivation, and finally obtain the target product I after beating with acetone (100ml), the yield is 75.6%, and the purity is 99.42%.
Embodiment 2
[0055] At room temperature, compound SM-1 (17.81g, 0.05mol), trifluridine (20.73g, 0.07mol), Novozyme435 (44.53g) was added to ethanol (200ml), and the temperature was controlled at 25-30°C to react. After completion, add KOH (8.42g, 0.15mol) purified water (30ml) solution at temperature control 15-20°C. After adding, continue to react at temperature control 15-20°C. Concentrate under reduced pressure to dryness after sexing, and finally obtain the target product I after beating with acetone (100ml), the yield is 72.3%, and the purity is 99.10%.
Embodiment 3
[0057] At room temperature, compound SM-1 (17.81g, 0.05mol), trifluridine (59.24g, 0.20mol), and Novozyme435 (44.53g) were added to acetone (200ml), and the temperature was controlled at 25-30°C to react. After completion, add KOH (8.42g, 0.15mol) purified water (30ml) solution at 15-20°C temperature control, continue to control temperature at 0-5°C for reaction, and adjust the pH of the reaction solution to neutral after the reaction is completed. Concentrate under reduced pressure to dryness after deactivation, and finally obtain the target product I after beating with acetone (100ml), the yield is 71.5%, and the purity is 99.16%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com