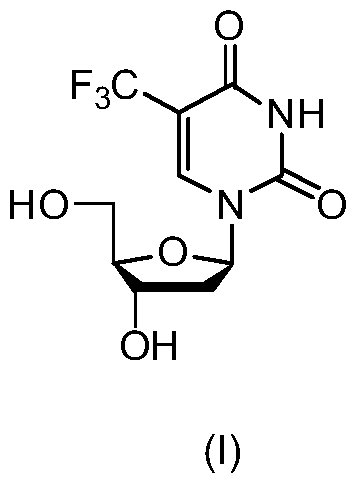
New crystal form of trifluridine and preparation method thereof
A crystal form and compound technology, applied in the field of medicinal chemistry, can solve the problems of no crystal form patent report of trifluridine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of Trifluridine Form I Using Acetone / Isopropyl Ether
[0021] Compound 1 (29.6g, 0.1mol) was added to acetone (300ml), heated to reflux, isopropyl ether (1L) was added dropwise, and then stirred at 50°C for 12h for crystallization. After filtration, the resulting solid was dried under reduced pressure at 50°C to constant weight. The target product (10.2 g, off-white solid) was obtained with a yield of 34.4%.
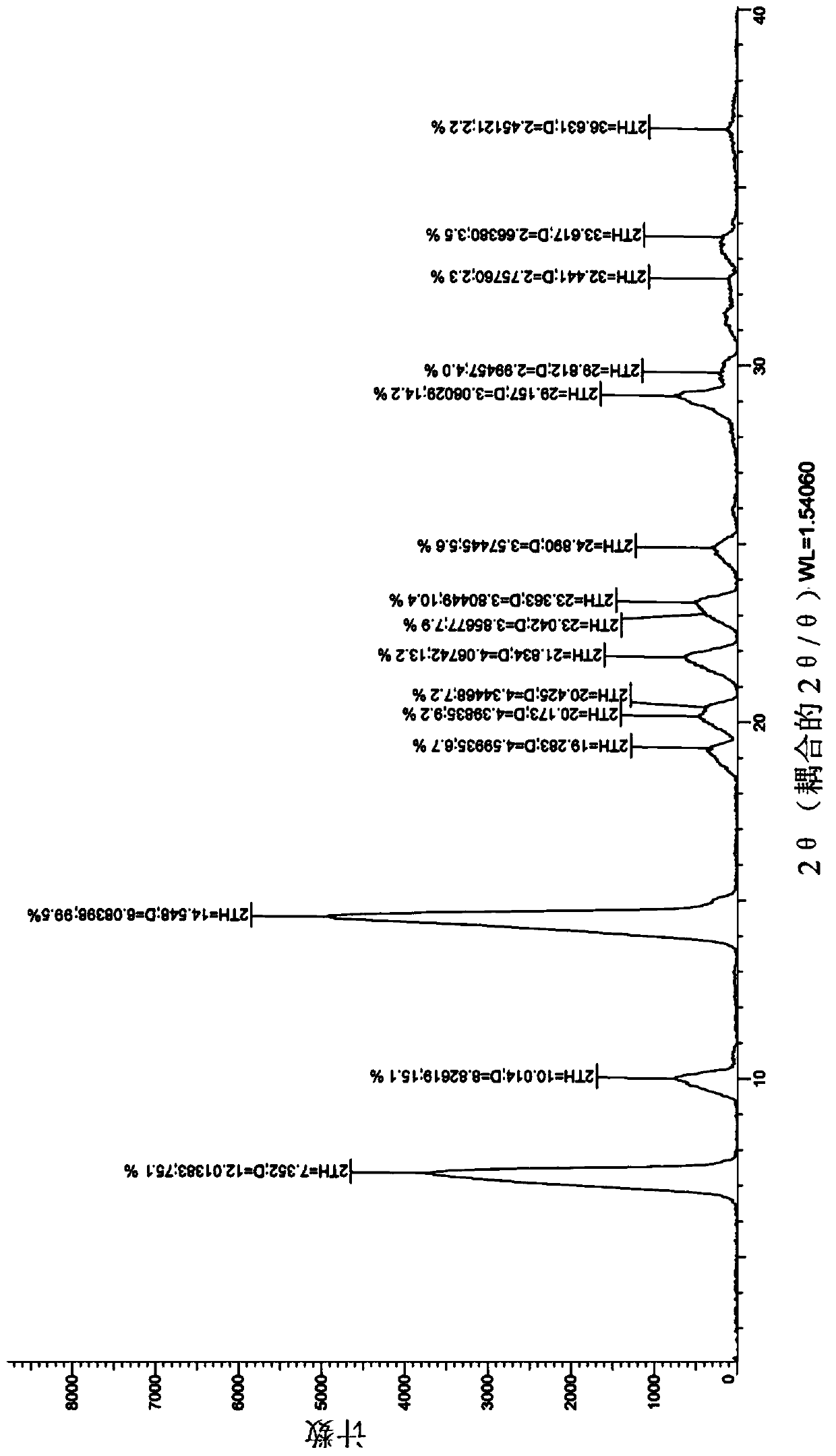
[0022] figure 1 It is the X-ray diffraction figure of the crystal form I obtained in Example 1.
experiment example 1
[0023] Experimental Example 1 Stability Experiment
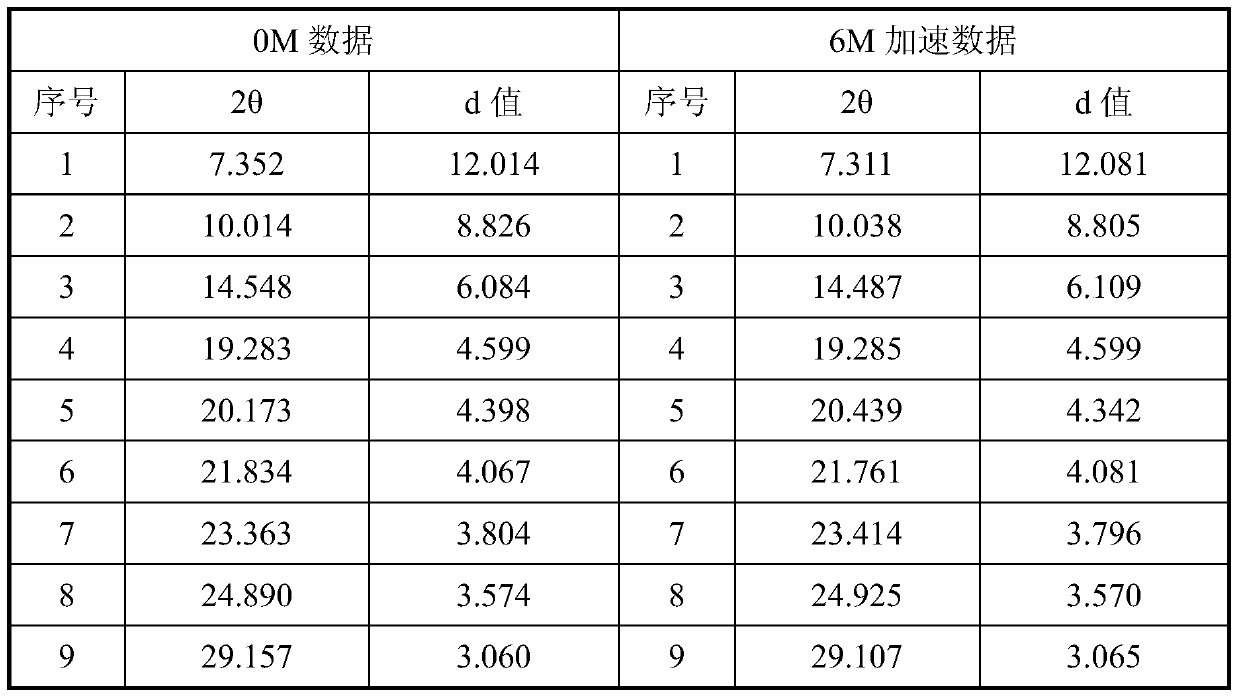
[0024] The accelerated stability study was carried out on the crystal form prepared in Example 1, and the X-ray diffraction data comparison between 0M and 6M in the accelerated stability test is shown in Table 1.
[0025] Table 1 X-ray diffraction data comparison table of accelerated stability test samples
[0026]
[0027] Experimental conclusion: after 6 months of accelerated experimentation, the X-ray diffraction spectrum is consistent with the initial data, and no crystal transformation occurs, indicating that the crystal form provided by the present invention has good stability.
experiment example 2
[0028] Experimental Example 2 Stability Experiment
[0029] Table 2 Stability test comparison table
[0030]
[0031] Experimental conclusion: the crystal form provided by the present invention has good stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com