METHOD FOR DETECTING similar SUBSTANCE derived from TRIFLURIDINE
A technology for trifluridine and substances, which is applied in the field of determination of similar substances in preparations, can solve problems such as unrecorded conditions and no published detection methods, and achieves the effect of ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0189] Column: Hydrosphere C18, manufactured by YMC Corporation (3 μm)
[0190] Flow rate: 1.0mL / min
[0191] Mobile phase A: 0.05mol / L sodium dihydrogen phosphate aqueous solution
[0192] Mobile Phase B: Acetonitrile
[0193] Gradient: Change the mobile phase A and mobile phase B in the following way to control the concentration gradient. 0~18 minutes: A 95%, B5%; 18~55 minutes: A 95→60%, B 5%→40%; 55~55.1 minutes: A 60%→95%, B 40%→5%; 55.1 Minutes later: A 95%, B 5%.
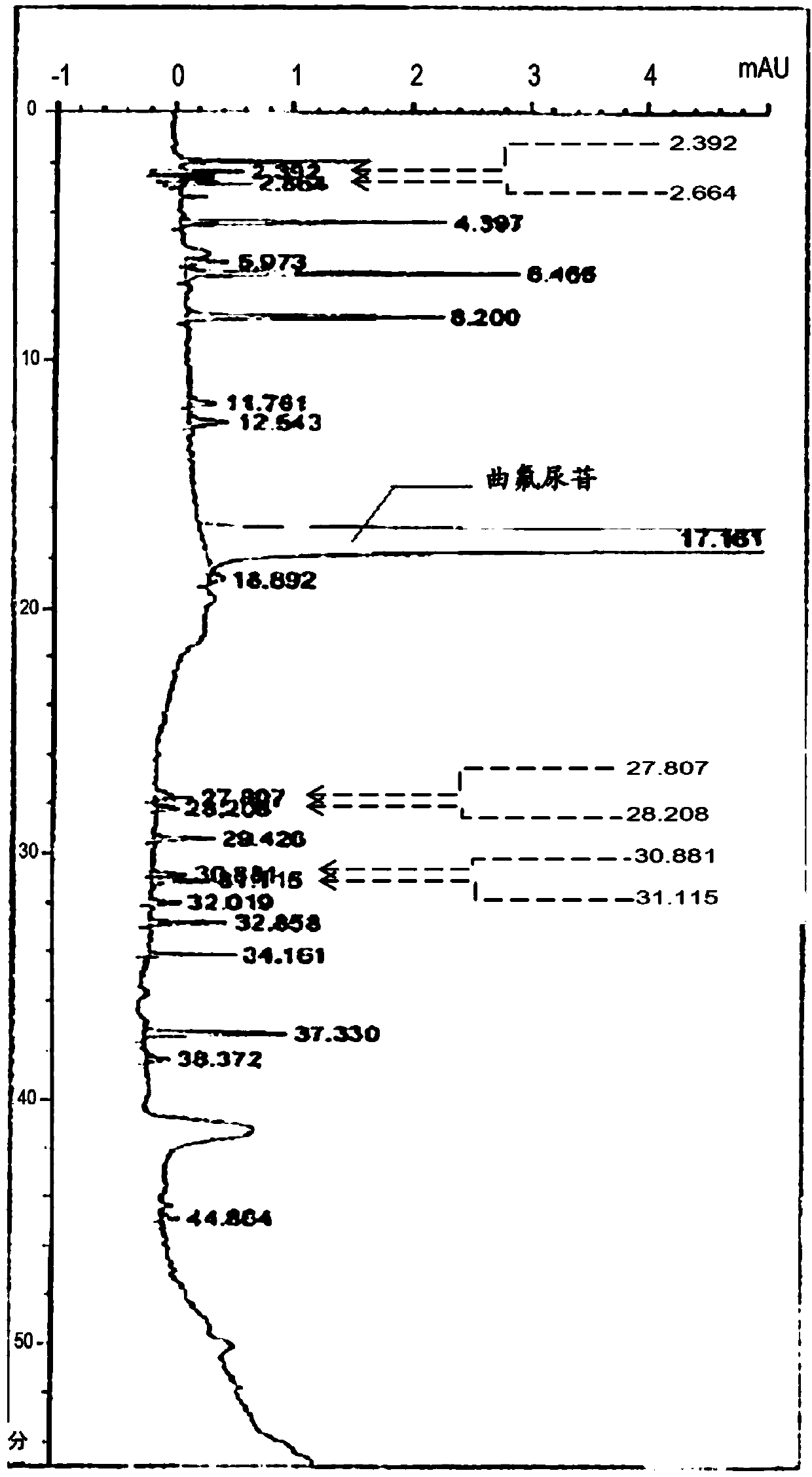
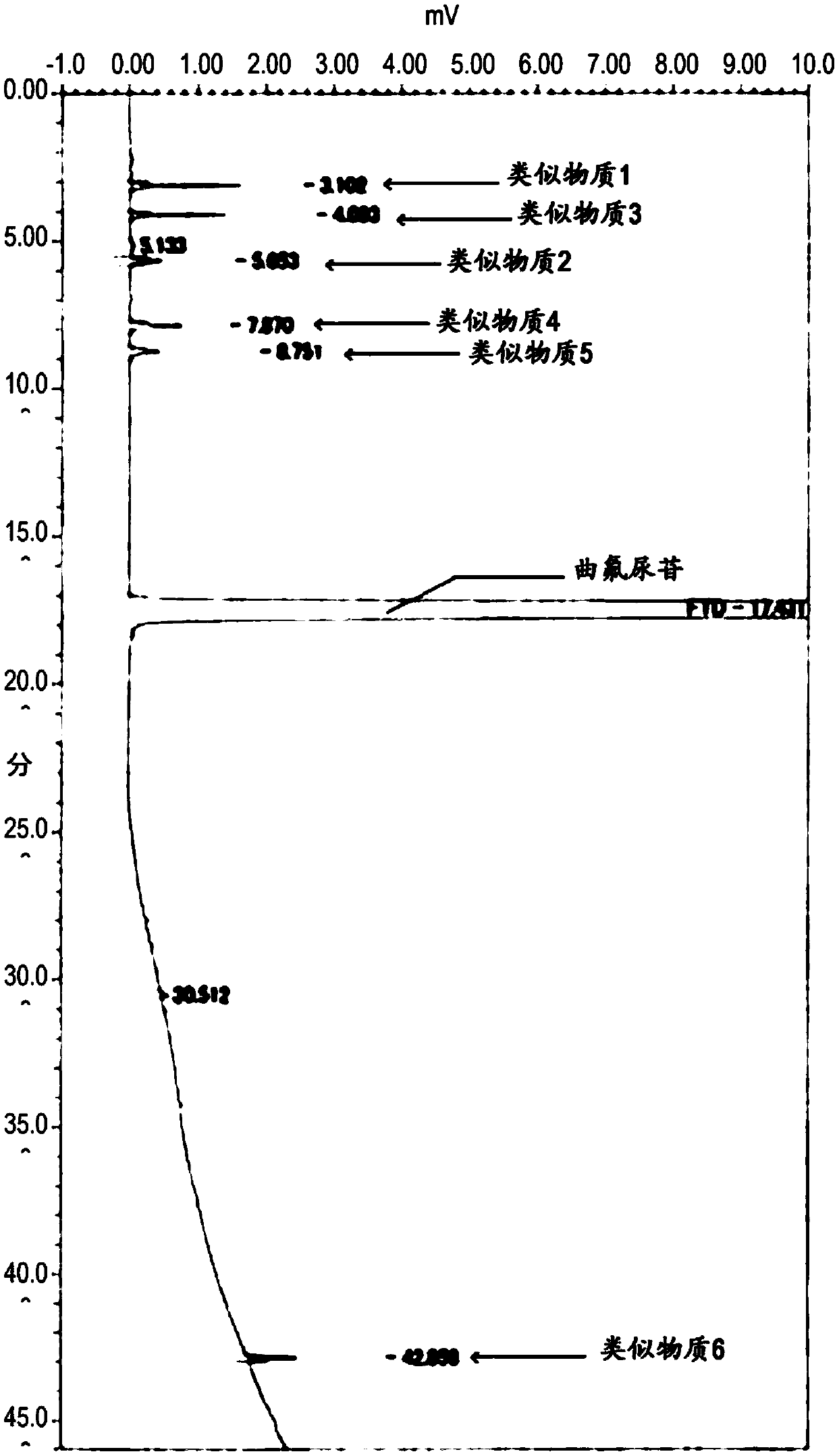
[0194]The measurement results are shown in figure 1 . From this, it was confirmed that the retention time of trifluridine was 17.2 minutes. In this measurement method, it was confirmed that trifluridine did not overlap with dips or false peaks in the baseline.
Embodiment 1-2
[0196] Gradient: Change the mobile phase A and mobile phase B in the following way to control the concentration gradient. 0~18 minutes: A 95%, B5%; 18~48 minutes: A 95→60%, B 5%→40%; 48~48.1 minutes: A 60%→95%, B 40%→5%; 48.1 Minutes later: A 95%, B 5%.
[0197] Chromatographic column, flow rate, mobile phase A and mobile phase B are the same conditions as in Example 1.
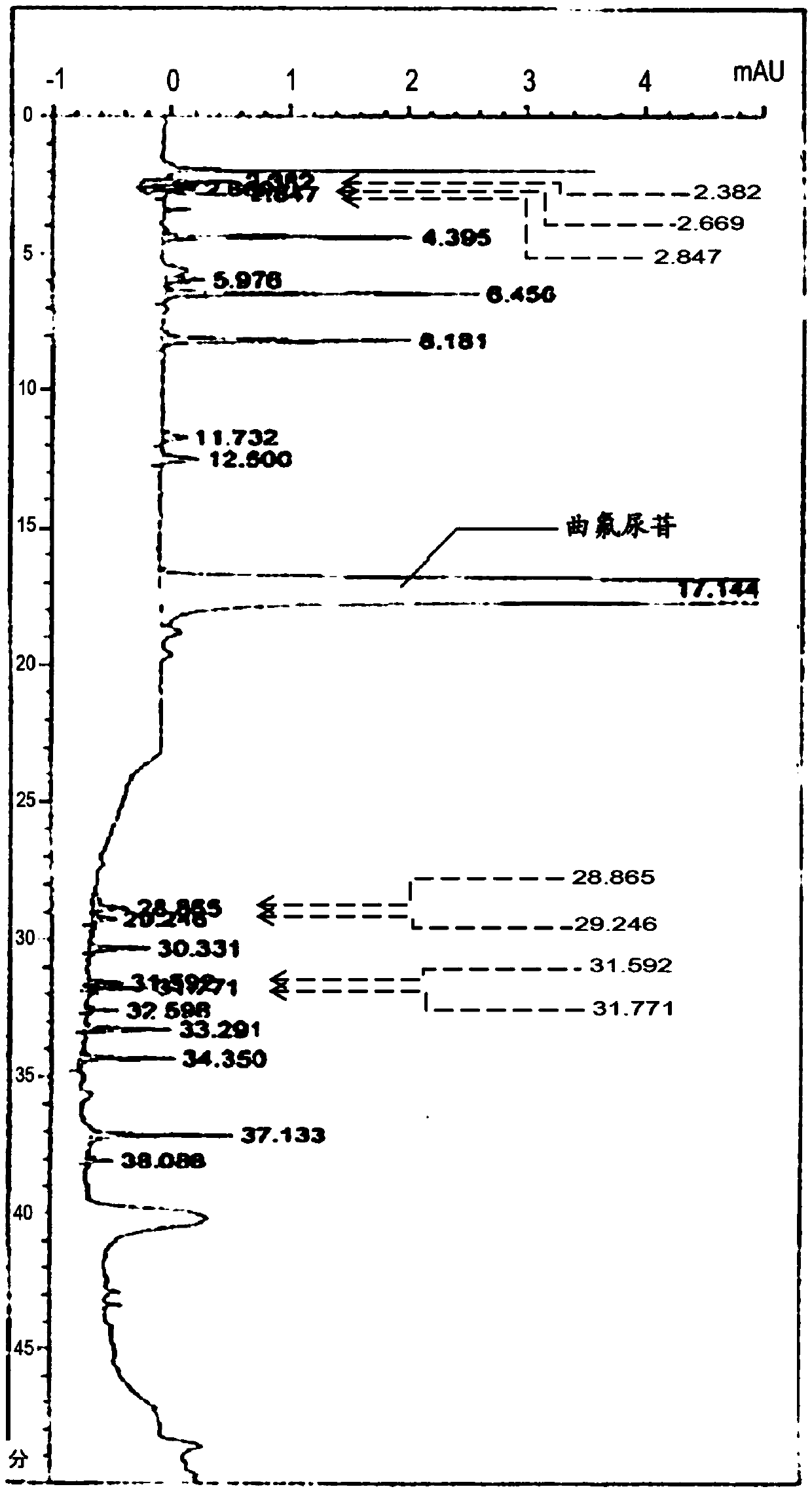
[0198] By this measurement, it was confirmed that trifluridine did not overlap with false peaks.
Embodiment 1-3
[0200] Gradient: Change the mobile phase A and mobile phase B in the following way to control the concentration gradient. 0-20 minutes: A 95%, B5%; 20-50 minutes: A 95→60%, B 5%→40%; 50-50.1 minutes: A 60%→95%, B 40%→5%; 50.1 Minutes later: A 95%, B 5%. Chromatographic column, flow rate, mobile phase A and mobile phase B are the same conditions as in Example 1.
[0201] The measurement results are shown in figure 2 . From this, it was confirmed that the retention time of trifluridine was 17.1 minutes. In this measurement method, it was confirmed that trifluridine did not overlap with dips or false peaks in the baseline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com