Application of trifluridine in preparation of HIV-1 drug
A technology of HIV-1 and trifluridine, applied in the field of HIV-1, can solve the problems of activation and killing obstacles, toxic and side effects, etc., and achieve good activation effect, low toxicity and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
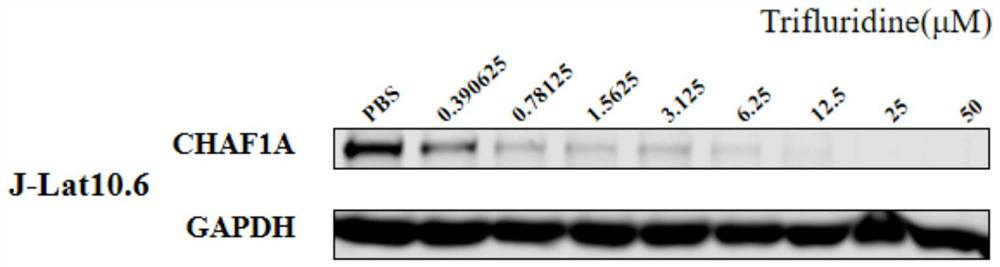
[0031] Example 1 Degradation experiment of CHAF1A by trifluridine in J-Lat 10.6 cell line.
[0032] The inventor's previous research has found that the host protein CHAF1A is a key factor in the formation and maintenance of HIV-1 latency, and is a safe and effective HIV-1 latent infection activation target. Therefore, the degradation effect of different concentrations of trifluridine on CHAF1A was detected to confirm the most appropriate drug concentration.
[0033] 1. Experimental method
[0034] Take an appropriate amount of well-growing J-Lat 10.6 cells and plate them. The medium used: 1640 medium, 10% fetal bovine serum and 1% penicillin-streptomycin mixed solution, culture condition: 5% carbon dioxide, 37°C.
[0035] Add final concentrations of 50 μM, 25 μM, 12.5 μM, 6.25 μM, 3.125 μM, 1.5625 μM, 0.78125 μM, 0.390625 μM trifluridine into the medium to treat the cells, and the PBS treatment group was used as a control, and the samples were collected for Western Blot dete...
Embodiment 2 3
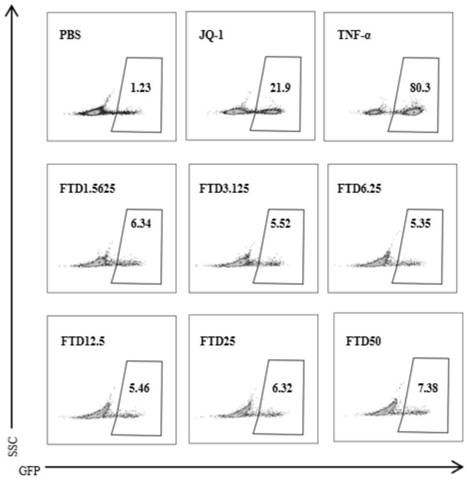
[0038] Example 2 The activation effect of trifluridine on latent infection in J-Lat 10.6 cell line
[0039] 1. Experimental method
[0040] 1) Well-grown J-Lat 10.6 cells were plated, the medium used: 1640 medium, 10% fetal bovine serum and 1% penicillin-streptomycin mixed solution, culture conditions: 5% carbon dioxide, 37°C.
[0041] 5 cells per well in a 2-well clear plate; add trifluridine at a final concentration of 50 μM, 25 μM, 12.5 μM, 6.25 μM, and 3.125 μM, respectively, use PBS as a negative control, and take TNF-α treatment group and JQ-1 treatment group as positive control group.
[0042] 2) Cultivate for 48 hours, collect the cells by centrifugation, discard the supernatant, wash once with PBS, discard the supernatant, and then resuspend with PBS;
[0043] 3) Use a flow cytometer to detect the GFP expression level of the corresponding cells, and make a statistical graph to analyze the results.
[0044] 2. Experimental results
[0045] Experimental results such...
Embodiment 3
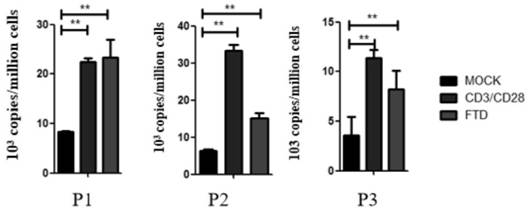
[0046] Example 3 Fluoxuridine Activation of HIV-1 in Clinical Samples of Infected Persons
[0047] 1. Experimental method
[0048] 1) Well-grown J-Lat cells were plated, the medium used: 1640 medium, 10% fetal bovine serum and 1% penicillin-streptomycin mixed solution, culture conditions: 5% carbon dioxide, 37°C. Different concentrations of trifluridine were added, and the final concentrations were: 100 μM, 25 μM, 6.25 μM, 1.5625 μM, .0390625 μM, 0.097656 μM, 0.024414 μM, 0.006104 μM, 0.001526 μM, 0.000381 μM, 0 μM.
[0049] 2) Cultivate for 48 hours, collect the cells by centrifugation, discard the supernatant, wash once with PBS, discard the supernatant, and then resuspend with PBS;
[0050] 3) Use a flow cytometer to detect the GFP expression level of the corresponding cells, and make a statistical graph to analyze the results.
[0051] 2. Experimental results
[0052] Experimental results such as Figure 4 shown. It can be seen from the experimental results that the E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com