Preparation method of high purity trifluridine
A high-purity technology of trifluridine, which is applied in the field of medicine, can solve the problems of high operating cost, low purity, and low yield, and achieve the effects of simplifying the process flow, clear route, and improving purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
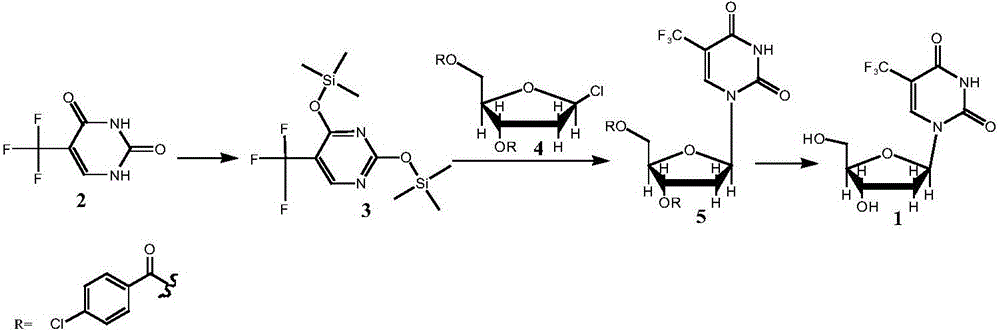
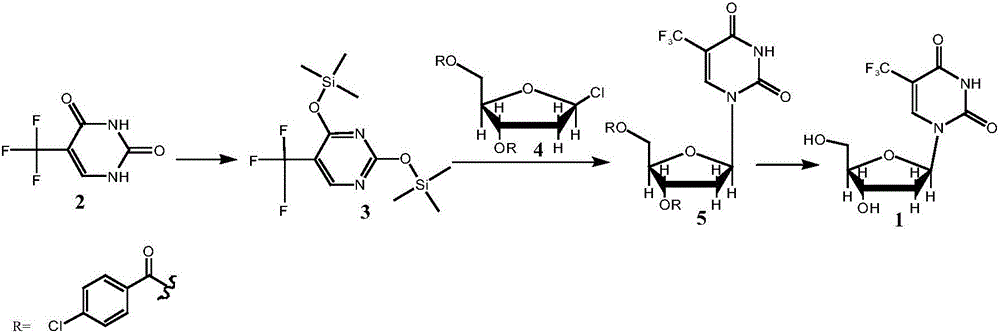
[0037] Preparation of Compound 3: At room temperature, add 90ml of HMDS and 2ml of TMSCl into the reactor, add 18g of Compound 2 (5-trifluoromethyluracil) under stirring, raise the temperature to 125°C, stir for 2h, and cool to 60°C , and concentrated under reduced pressure at 70-80°C until no liquid flowed out to obtain 30 g of light yellow liquid with a yield of 92.5%, HPLC: 96%.
[0038] Preparation of Compound 5: At room temperature, add 16.4g of Compound 3 to 200ml of chloroform, and add 21.4g of Compound 4 (1-chloro-3,5-di-p-chlorobenzoyl-2-deoxy-D-ribose) under stirring With 0.5g copper difluoride, react at 20-30°C for 24 hours after the addition, add 60ml 1mol / L hydrochloric acid solution to the system after the reaction, stir for 10min, separate the liquid, wash the organic phase twice with 100ml water / time, concentrate The organic phase flows out until there is no solvent, and the residue is recrystallized by adding 300ml of ethanol, the crystals are filtered out, ri...
Embodiment 2
[0041] Preparation of Compound 5: At room temperature, add 19.7g of Compound 3 to 200ml of chloroform, and add 21.4g of Compound 4 (1-chloro-3,5-di-p-chlorobenzoyl-2-deoxy-D-ribose) under stirring With 2.5g copper difluoride, react at 20-30°C for 24 hours after the addition, add 60ml 1mol / L hydrochloric acid solution to the system after the reaction, stir for 10min, separate the liquid, wash the organic phase twice with 100ml water / time, concentrate The organic phase flows out until there is no solvent, the residue is recrystallized by adding 320ml of ethanol, the crystals are filtered out, rinsed with an appropriate amount of ethanol, and the filter cake is vacuum-dried at 45°C for 6h to obtain 23.0g of a white solid, with a yield of 80.2%, HPLC: 96.5% .
[0042] Preparation of Compound 1: Add 11.5g of Compound 5 to 80ml of methanol at room temperature, add 3.3g of sodium methoxide under stirring, stir and react at 20-30°C for 4h, add 4.2g of strong acidic cationic resin and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com